Ecology and Genetic Analysis of Katablepharis CRE, A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Report Is Also Available in Adobe
U.S. Department of the Interior U.S. Geological Survey Water Quality of Rob Roy Reservoir and Lake Owen, Albany County, and Granite Springs and Crystal Lake Reservoirs, Laramie County, Wyoming, 1997-98 By Kathy Muller Ogle1, David A. Peterson1, Bud Spillman2, and Rosie Padilla2 Water-Resources Investigations Report 99-4220 Prepared in cooperation with the Cheyenne Board of Public Utilities 1U.S. Geological Survey 2Cheyenne Board of Public Utilities Cheyenne, Wyoming 1999 U.S. Department of the Interior Bruce Babbitt, Secretary U.S. Geological Survey Charles G. Groat, Director Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government For additional information write to: District Chief U.S. Geological Survey, WRD 2617 E. Lincolnway, Suite B Cheyenne, Wyoming 82001-5662 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286, Denver Federal Center Denver, Colorado 80225 Information regarding the programs of the Wyoming District is available on the Internet via the World Wide Web. You may connect to the Wyoming District Home Page using the Universal Resource Locator (URL): http://wy.water.usgs.gov CONTENTS Page Abstract ............................................................................................................................................................................... 1 Introduction........................................................................................................................................................................ -

Sex Is a Ubiquitous, Ancient, and Inherent Attribute of Eukaryotic Life
PAPER Sex is a ubiquitous, ancient, and inherent attribute of COLLOQUIUM eukaryotic life Dave Speijera,1, Julius Lukešb,c, and Marek Eliášd,1 aDepartment of Medical Biochemistry, Academic Medical Center, University of Amsterdam, 1105 AZ, Amsterdam, The Netherlands; bInstitute of Parasitology, Biology Centre, Czech Academy of Sciences, and Faculty of Sciences, University of South Bohemia, 370 05 Ceské Budejovice, Czech Republic; cCanadian Institute for Advanced Research, Toronto, ON, Canada M5G 1Z8; and dDepartment of Biology and Ecology, University of Ostrava, 710 00 Ostrava, Czech Republic Edited by John C. Avise, University of California, Irvine, CA, and approved April 8, 2015 (received for review February 14, 2015) Sexual reproduction and clonality in eukaryotes are mostly Sex in Eukaryotic Microorganisms: More Voyeurs Needed seen as exclusive, the latter being rather exceptional. This view Whereas absence of sex is considered as something scandalous for might be biased by focusing almost exclusively on metazoans. a zoologist, scientists studying protists, which represent the ma- We analyze and discuss reproduction in the context of extant jority of extant eukaryotic diversity (2), are much more ready to eukaryotic diversity, paying special attention to protists. We accept that a particular eukaryotic group has not shown any evi- present results of phylogenetically extended searches for ho- dence of sexual processes. Although sex is very well documented mologs of two proteins functioning in cell and nuclear fusion, in many protist groups, and members of some taxa, such as ciliates respectively (HAP2 and GEX1), providing indirect evidence for (Alveolata), diatoms (Stramenopiles), or green algae (Chlor- these processes in several eukaryotic lineages where sex has oplastida), even serve as models to study various aspects of sex- – not been observed yet. -

Supporting Information
Supporting Information Parker et al. 10.1073/pnas.0806481105 Table S1. Species abbreviations used in Fig. 4A Major taxon Species Abbreviation Major taxon Species Abbreviation Dinobryon cylindraceum Dino cyli Diatoms Achnanthes flexella Achn flex Dinobryon sociale Dino soci Achnanthes lanceolata Dinobryon sp. (monad) Achnanthes minutissima Kephyrion boreale Keph bore Cocconeis placentula Kephyrion sp. Cyclotella atomus Cycl atom Mallomonas sp. Cyclotella bodanica Cycl boda Ochromonas minima Ochr mini Cyclotella comensis Cycl come Ochromonas sp. Cyclotella glomerata Pseudopedinella sp. Cyclotella ocellata Cycl ocel Salpingoeca sp. Cyclotella sp. Synura sp. Cymbella arctica Cymb arct Cymbella descripta Cymb desc Cryptophytes Cryptomonas erosa Cryp eros Cymbella minuta Cymb minu Cryptomonas marsonii Cryp mars Cymbella sp. Cryptomonas ovata Denticula subtilis Dent subt Cryptomonas platyrius Cryp plat Diatoma vulgare Diat vulg Cryptomonas reflexa Cryp refl Fragilaria capucina Frag capu Cryptomonas rostratiformis Cryp rost Fragilaria construens Cryptomonas sp. Fragilaria cyclopum Frag cycl Katablepharis ovalis Kata oval Fragilaria filiformis Rhodomonas lens Rhod lens Fragilaria tenera Rhodomonas minuta Rhod minu Fragilaria pinnata Frag pinn Gomphonema parvulum Gomp parv Dinoflagellates Amphidinium sp Gomphonema sp. Gymnodinium fungiforme Gymn fung Meridion circulare Meri circ Gymnodinium fuscum Navicula cryocephala Gymnodinium helvetica Gymn helv Navicula pupula Gymnodinium inversum Gymn inve Nitzschia perminuta Gymnodinium lacustre Gymn lacu Nitzschia -

An Integrative Approach Sheds New Light Onto the Systematics
www.nature.com/scientificreports OPEN An integrative approach sheds new light onto the systematics and ecology of the widespread ciliate genus Coleps (Ciliophora, Prostomatea) Thomas Pröschold1*, Daniel Rieser1, Tatyana Darienko2, Laura Nachbaur1, Barbara Kammerlander1, Kuimei Qian1,3, Gianna Pitsch4, Estelle Patricia Bruni4,5, Zhishuai Qu6, Dominik Forster6, Cecilia Rad‑Menendez7, Thomas Posch4, Thorsten Stoeck6 & Bettina Sonntag1 Species of the genus Coleps are one of the most common planktonic ciliates in lake ecosystems. The study aimed to identify the phenotypic plasticity and genetic variability of diferent Coleps isolates from various water bodies and from culture collections. We used an integrative approach to study the strains by (i) cultivation in a suitable culture medium, (ii) screening of the morphological variability including the presence/absence of algal endosymbionts of living cells by light microscopy, (iii) sequencing of the SSU and ITS rDNA including secondary structures, (iv) assessment of their seasonal and spatial occurrence in two lakes over a one‑year cycle both from morphospecies counts and high‑ throughput sequencing (HTS), and, (v) proof of the co‑occurrence of Coleps and their endosymbiotic algae from HTS‑based network analyses in the two lakes. The Coleps strains showed a high phenotypic plasticity and low genetic variability. The algal endosymbiont in all studied strains was Micractinium conductrix and the mutualistic relationship turned out as facultative. Coleps is common in both lakes over the whole year in diferent depths and HTS has revealed that only one genotype respectively one species, C. viridis, was present in both lakes despite the diferent lifestyles (mixotrophic with green algal endosymbionts or heterotrophic without algae). -

A Resurgence in Field Research Is Essential to Better Understand
Received Date : 15-Mar-2013 Revised Date : 21-Oct-2013 Accepted Date : 29-Oct-2013 Article type : Symposium Article Heger et al. --- Importance of Field Protistology A Resurgence in Field Research is Essential to Better Understand the Diversity, Ecology, and Evolution of Microbial Eukaryotes Thierry J. Hegera, Virginia P. Edgcombb, Eunsoo Kimc, Julius Lukešd, Brian S. Leandera & Naoji Yubukia a The Departments of Botany and Zoology, Beaty Biodiversity Research Centre and Museum, University of British Columbia, Vancouver, British Columbia, V6T 1Z4, Canada b Woods Hole Oceanographic Institution, Geology and Geophysics Department, Woods Hole, Article MA 02543, USA c Division of Invertebrate Zoology, American Museum of Natural History, New York, NY, 10024, USA d Institute of Parasitology, Biology Centre, Czech Academy of Sciences, and Faculty of Science, University of South Bohemia, 37005 České Budějovice, Czech Republic Correspondence T. J. Heger and N. Yubuki, The Departments of Botany and Zoology, Beaty Biodiversity Research Centre and Museum, University of British Columbia, 6270 University Blvd., Vancouver, British Columbia, V6T 1Z4, Canada Telephone number: +1 604 822 4892; FAX number: +1 604 822 6089; emails: [email protected] and [email protected] ABSTRACT The discovery and characterization of protist communities from diverse environments are crucial for understanding the overall evolutionary history of life on earth. However, major questions about the diversity, ecology, and evolutionary history of protists remain unanswered, -

The Plankton Lifeform Extraction Tool: a Digital Tool to Increase The
Discussions https://doi.org/10.5194/essd-2021-171 Earth System Preprint. Discussion started: 21 July 2021 Science c Author(s) 2021. CC BY 4.0 License. Open Access Open Data The Plankton Lifeform Extraction Tool: A digital tool to increase the discoverability and usability of plankton time-series data Clare Ostle1*, Kevin Paxman1, Carolyn A. Graves2, Mathew Arnold1, Felipe Artigas3, Angus Atkinson4, Anaïs Aubert5, Malcolm Baptie6, Beth Bear7, Jacob Bedford8, Michael Best9, Eileen 5 Bresnan10, Rachel Brittain1, Derek Broughton1, Alexandre Budria5,11, Kathryn Cook12, Michelle Devlin7, George Graham1, Nick Halliday1, Pierre Hélaouët1, Marie Johansen13, David G. Johns1, Dan Lear1, Margarita Machairopoulou10, April McKinney14, Adam Mellor14, Alex Milligan7, Sophie Pitois7, Isabelle Rombouts5, Cordula Scherer15, Paul Tett16, Claire Widdicombe4, and Abigail McQuatters-Gollop8 1 10 The Marine Biological Association (MBA), The Laboratory, Citadel Hill, Plymouth, PL1 2PB, UK. 2 Centre for Environment Fisheries and Aquacu∑lture Science (Cefas), Weymouth, UK. 3 Université du Littoral Côte d’Opale, Université de Lille, CNRS UMR 8187 LOG, Laboratoire d’Océanologie et de Géosciences, Wimereux, France. 4 Plymouth Marine Laboratory, Prospect Place, Plymouth, PL1 3DH, UK. 5 15 Muséum National d’Histoire Naturelle (MNHN), CRESCO, 38 UMS Patrinat, Dinard, France. 6 Scottish Environment Protection Agency, Angus Smith Building, Maxim 6, Parklands Avenue, Eurocentral, Holytown, North Lanarkshire ML1 4WQ, UK. 7 Centre for Environment Fisheries and Aquaculture Science (Cefas), Lowestoft, UK. 8 Marine Conservation Research Group, University of Plymouth, Drake Circus, Plymouth, PL4 8AA, UK. 9 20 The Environment Agency, Kingfisher House, Goldhay Way, Peterborough, PE4 6HL, UK. 10 Marine Scotland Science, Marine Laboratory, 375 Victoria Road, Aberdeen, AB11 9DB, UK. -

Biovolumes and Size-Classes of Phytoplankton in the Baltic Sea
Baltic Sea Environment Proceedings No.106 Biovolumes and Size-Classes of Phytoplankton in the Baltic Sea Helsinki Commission Baltic Marine Environment Protection Commission Baltic Sea Environment Proceedings No. 106 Biovolumes and size-classes of phytoplankton in the Baltic Sea Helsinki Commission Baltic Marine Environment Protection Commission Authors: Irina Olenina, Centre of Marine Research, Taikos str 26, LT-91149, Klaipeda, Lithuania Susanna Hajdu, Dept. of Systems Ecology, Stockholm University, SE-106 91 Stockholm, Sweden Lars Edler, SMHI, Ocean. Services, Nya Varvet 31, SE-426 71 V. Frölunda, Sweden Agneta Andersson, Dept of Ecology and Environmental Science, Umeå University, SE-901 87 Umeå, Sweden, Umeå Marine Sciences Centre, Umeå University, SE-910 20 Hörnefors, Sweden Norbert Wasmund, Baltic Sea Research Institute, Seestr. 15, D-18119 Warnemünde, Germany Susanne Busch, Baltic Sea Research Institute, Seestr. 15, D-18119 Warnemünde, Germany Jeanette Göbel, Environmental Protection Agency (LANU), Hamburger Chaussee 25, D-24220 Flintbek, Germany Slawomira Gromisz, Sea Fisheries Institute, Kollataja 1, 81-332, Gdynia, Poland Siv Huseby, Umeå Marine Sciences Centre, Umeå University, SE-910 20 Hörnefors, Sweden Maija Huttunen, Finnish Institute of Marine Research, Lyypekinkuja 3A, P.O. Box 33, FIN-00931 Helsinki, Finland Andres Jaanus, Estonian Marine Institute, Mäealuse 10 a, 12618 Tallinn, Estonia Pirkko Kokkonen, Finnish Environment Institute, P.O. Box 140, FIN-00251 Helsinki, Finland Iveta Ledaine, Inst. of Aquatic Ecology, Marine Monitoring Center, University of Latvia, Daugavgrivas str. 8, Latvia Elzbieta Niemkiewicz, Maritime Institute in Gdansk, Laboratory of Ecology, Dlugi Targ 41/42, 80-830, Gdansk, Poland All photographs by Finnish Institute of Marine Research (FIMR) Cover photo: Aphanizomenon flos-aquae For bibliographic purposes this document should be cited to as: Olenina, I., Hajdu, S., Edler, L., Andersson, A., Wasmund, N., Busch, S., Göbel, J., Gromisz, S., Huseby, S., Huttunen, M., Jaanus, A., Kokkonen, P., Ledaine, I. -

Table of Contents
PLASTID-TARGETED PROTEINS ARE ABSENT FROM THE PROTEOMES OF ACHLYA HYPOGYNA AND THRAUSTOTHECA CLAVATA (OOMYCOTA, STRAMENOPILA): IMPLICATIONS FOR THE ORIGIN OF CHROMALVEOLATE PLASTIDS AND THE ‘GREEN GENE’ HYPOTHESIS Lindsay Rukenbrod A Thesis Submitted to the University of North Carolina Wilmington in Partial Fulfillment of the Requirements for the Degree of Master of Science Center for Marine Science University of North Carolina Wilmington 2012 Approved by Advisory Committee D. Wilson Freshwater Jeremy Morgan Allison Taylor J. Craig Bailey Chair Accepted by Dean, Graduate School This thesis has been prepared in the style and format consistent with the Journal of Eukaryotic Microbiology. ii TABLE OF CONTENTS ABSTRACT .....................................................................................................................iv ACKNOWLEDGMENTS ..................................................................................................vi DEDICATION ................................................................................................................. vii LIST OF TABLES .......................................................................................................... viii LIST OF FIGURES ..........................................................................................................ix CHAPTER 1: Implications for the origin of chromalveolate plastids ............................... X INTRODUCTION .................................................................................................. 1 METHODS........................................................................................................... -

Nuclear Genome Sequence of the Plastid-Lacking
Cenci et al. BMC Biology (2018) 16:137 https://doi.org/10.1186/s12915-018-0593-5 RESEARCH ARTICLE Open Access Nuclear genome sequence of the plastid- lacking cryptomonad Goniomonas avonlea provides insights into the evolution of secondary plastids Ugo Cenci1,2†, Shannon J. Sibbald1,2†, Bruce A. Curtis1,2, Ryoma Kamikawa3, Laura Eme1,2,11, Daniel Moog1,2,12, Bernard Henrissat4,5,6, Eric Maréchal7, Malika Chabi8, Christophe Djemiel8, Andrew J. Roger1,2,9, Eunsoo Kim10 and John M. Archibald1,2,9* Abstract Background: The evolution of photosynthesis has been a major driver in eukaryotic diversification. Eukaryotes have acquired plastids (chloroplasts) either directly via the engulfment and integration of a photosynthetic cyanobacterium (primary endosymbiosis) or indirectly by engulfing a photosynthetic eukaryote (secondary or tertiary endosymbiosis). The timing and frequency of secondary endosymbiosis during eukaryotic evolution is currently unclear but may be resolved in part by studying cryptomonads, a group of single-celled eukaryotes comprised of both photosynthetic and non-photosynthetic species. While cryptomonads such as Guillardia theta harbor a red algal-derived plastid of secondary endosymbiotic origin, members of the sister group Goniomonadea lack plastids. Here, we present the genome of Goniomonas avonlea—the first for any goniomonad—to address whether Goniomonadea are ancestrally non-photosynthetic or whether they lost a plastid secondarily. Results: We sequenced the nuclear and mitochondrial genomes of Goniomonas avonlea and carried out a comparative analysis of Go. avonlea, Gu. theta, and other cryptomonads. The Go. avonlea genome assembly is ~ 92 Mbp in size, with 33,470 predicted protein-coding genes. Interestingly, some metabolic pathways (e.g., fatty acid biosynthesis) predicted to occur in the plastid and periplastidal compartment of Gu. -

EEF2 Analysis Challenges the Monophyly of Archaeplastida and Chromalveolata
EEF2 Analysis Challenges the Monophyly of Archaeplastida and Chromalveolata Eunsoo Kim¤*, Linda E. Graham Department of Botany, University of Wisconsin-Madison, Madison, Wisconsin, United States of America Abstract Background: Classification of eukaryotes provides a fundamental phylogenetic framework for ecological, medical, and industrial research. In recent years eukaryotes have been classified into six major supergroups: Amoebozoa, Archaeplastida, Chromalveolata, Excavata, Opisthokonta, and Rhizaria. According to this supergroup classification, Archaeplastida and Chromalveolata each arose from a single plastid-generating endosymbiotic event involving a cyanobacterium (Archaeplastida) or red alga (Chromalveolata). Although the plastids within members of the Archaeplastida and Chromalveolata share some features, no nucleocytoplasmic synapomorphies supporting these supergroups are currently known. Methodology/Principal Findings: This study was designed to test the validity of the Archaeplastida and Chromalveolata through the analysis of nucleus-encoded eukaryotic translation elongation factor 2 (EEF2) and cytosolic heat-shock protein of 70 kDa (HSP70) sequences generated from the glaucophyte Cyanophora paradoxa, the cryptophytes Goniomonas truncata and Guillardia theta, the katablepharid Leucocryptos marina, the rhizarian Thaumatomonas sp. and the green alga Mesostigma viride. The HSP70 phylogeny was largely unresolved except for certain well-established groups. In contrast, EEF2 phylogeny recovered many well-established eukaryotic groups -
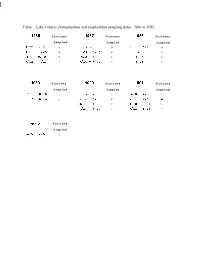
Table 1. Lake Ontario Phytoplankton and Zooplankton Sampling Dates, 1986 to 1992
Table 1. Lake Ontario phytoplankton and zooplankton sampling dates, 1986 to 1992. 1986 Stations 1987 Stations 1988 Stations Sampled Sampled Sampled 4/20 - 4/21 8 4/21 8 4/11 - 4/12 8 4/24 - 4/25 8 4/23 - 4/24 8 4/13 8 8/9 - 8/10 8 8/2 - 8/3 8 8/14 8 E/12 - E/13 8 8/14 - 8/15 8 8/16 8 1989 Stations 1990 Stations 1991 Stations Sampled Sampled Sampled 8/15 - 8/16 8 4/13 8 4/8 - 4/9 8 B/17 - 8/18 8 4/14 - 4/15 8 4/11 - 4/13 8 8/11 - 8/12 8 8/10 - B/11 8 8/13 - 8/14 8 E/12 - 8/13 8 1992 Stations Sampled 4/5 - 4/6 8 34 Table 2. Number of species observed in each algal division or grouping, Lake Ontario, 1986 to 1992. Spring and summer data only. BAC=Bacillariophyta, CHL=Chlorophyta, CHR=Chrysophyta, COL - colorless flagellates, CRY=Cryptophtya, CYA=Cyanophyta, EUG = Euglenophyta, PYR=Pyrrophyta, UN1 = unidentified flagellates. NUMBER OF SPECIES 1987 1988 1989 1990 1991 1992 1986-92 70 65 50 56 72 25 143 43 28 44 43 51 16 116 24 20 26 27 18 10 54 2 2 1 2 2 2 5 17 11 10 16 17 15 26 7 4 13 11 9 5 19 0 0 1 2 0 0 3 3 4 6 5 5 3 11 0 1 0 0 0 0 2 TOTAL 168 166 135 151 162 174 76 379 - -. -

Nusuttodinium Aeruginosum/Acidotum As a Case Study
Acquisition of Photoautotrophy in Kleptoplastic Dinoflagellates – Nusuttodinium aeruginosum/acidotum as a case study I n a u g u r a l – D i s s e r t a t i o n zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Universität zu Köln vorgelegt von Sebastian Wittek aus Krefeld 2018 Berichterstatter: Prof. Dr. Michael Melkonian Prof. Dr. Hartmut Arndt Prof. Dr. John M. Archibald Tag der mündlichen Prüfung: 16.01.2017 Kurzzusammenfassung in deutscher Sprache ------------------------------------------------------------------------------------------------------------------------------ Kurzzusammenfassung in deutscher Sprache Die Integrierung stabiler Plastiden durch Endosymbiosen führte zu einer enormen Vielfalt an photosynthetischen eukaryotischen Organismen auf der Erde. Allerdings sind die Schritte während der Etablierung stabiler Endosymbiosen nur schlecht verstanden. Um Licht auf diesen frühen Schritt der Evolution zu werfen, wurden viele Studien an Organismen mit vorübergehenden Plastiden durchgeführt. Ursprünglich heterotroph, sind diese Organismen in der Lage, Photoautotrophie durch die Aufnahme photosynthetischer Beute zu erwerben. Anstatt verdaut zu werden, behält die Beute ihre Fähigkeit zur Photosynthese bei und versorgt den Wirt mit Photosyntheseprodukten. Der Beuteorganismus kann entweder zu einem Endosymbiont oder sogar zu einem Plastid reduziert werden. Im letzteren Fall werden die Plastiden der photosynthetischen Beute gestohlen und daher ‚Kleptoplastiden‘ genannt. Kleptoplastiden sind innerhalb der Eukaryoten weit verbreitet und kommen in vielzelligen, wie auch in einzelligen Organismen vor. Eine der bekanntesten Gruppen, welche Kleptoplastiden beherbergt, sind die Dinoflagellaten. Diese Studie fokussiert auf Süßwasserisolate der Kleptoplastiden beherbergenden Gattung Nusuttodinium mit einem besonderen Fokus auf die Art N. aeruginosum/acidotum, welche ihre Kleptoplastiden von der blau-grünen cryptophytischen Gattung Chroomonas erlangt. Es wurden von N. aeruginosum/acidotum, sowie einer weiteren Art, N.