INTRODUCTION to PARTICLE PHYSICS from Atoms to Quarks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -

Fundamentals of Particle Physics
Fundamentals of Par0cle Physics Particle Physics Masterclass Emmanuel Olaiya 1 The Universe u The universe is 15 billion years old u Around 150 billion galaxies (150,000,000,000) u Each galaxy has around 300 billion stars (300,000,000,000) u 150 billion x 300 billion stars (that is a lot of stars!) u That is a huge amount of material u That is an unimaginable amount of particles u How do we even begin to understand all of matter? 2 How many elementary particles does it take to describe the matter around us? 3 We can describe the material around us using just 3 particles . 3 Matter Particles +2/3 U Point like elementary particles that protons and neutrons are made from. Quarks Hence we can construct all nuclei using these two particles -1/3 d -1 Electrons orbit the nuclei and are help to e form molecules. These are also point like elementary particles Leptons We can build the world around us with these 3 particles. But how do they interact. To understand their interactions we have to introduce forces! Force carriers g1 g2 g3 g4 g5 g6 g7 g8 The gluon, of which there are 8 is the force carrier for nuclear forces Consider 2 forces: nuclear forces, and electromagnetism The photon, ie light is the force carrier when experiencing forces such and electricity and magnetism γ SOME FAMILAR THE ATOM PARTICLES ≈10-10m electron (-) 0.511 MeV A Fundamental (“pointlike”) Particle THE NUCLEUS proton (+) 938.3 MeV neutron (0) 939.6 MeV E=mc2. Einstein’s equation tells us mass and energy are equivalent Wave/Particle Duality (Quantum Mechanics) Einstein E -

Introduction to Particle Physics
SFB 676 – Projekt B2 Introduction to Particle Physics Christian Sander (Universität Hamburg) DESY Summer Student Lectures – Hamburg – 20th July '11 Outline ● Introduction ● History: From Democrit to Thomson ● The Standard Model ● Gauge Invariance ● The Higgs Mechanism ● Symmetries … Break ● Shortcomings of the Standard Model ● Physics Beyond the Standard Model ● Recent Results from the LHC ● Outlook Disclaimer: Very personal selection of topics and for sure many important things are left out! 20th July '11 Introduction to Particle Physics 2 20th July '11 Introduction to Particle PhysicsX Files: Season 2, Episode 233 … für Chester war das nur ein Weg das Geld für das eigentlich theoretische Zeugs aufzubringen, was ihn interessierte … die Erforschung Dunkler Materie, …ähm… Quantenpartikel, Neutrinos, Gluonen, Mesonen und Quarks. Subatomare Teilchen Die Geheimnisse des Universums! Theoretisch gesehen sind sie sogar die Bausteine der Wirklichkeit ! Aber niemand weiß, ob sie wirklich existieren !? 20th July '11 Introduction to Particle PhysicsX Files: Season 2, Episode 234 The First Particle Physicist? By convention ['nomos'] sweet is sweet, bitter is bitter, hot is hot, cold is cold, color is color; but in truth there are only atoms and the void. Democrit, * ~460 BC, †~360 BC in Abdera Hypothesis: ● Atoms have same constituents ● Atoms different in shape (assumption: geometrical shapes) ● Iron atoms are solid and strong with hooks that lock them into a solid ● Water atoms are smooth and slippery ● Salt atoms, because of their taste, are sharp and pointed ● Air atoms are light and whirling, pervading all other materials 20th July '11 Introduction to Particle Physics 5 Corpuscular Theory of Light Light consist out of particles (Newton et al.) ↕ Light is a wave (Huygens et al.) ● Mainly because of Newtons prestige, the corpuscle theory was widely accepted (more than 100 years) Sir Isaac Newton ● Failing to describe interference, diffraction, and *1643, †1727 polarization (e.g. -

Read About the Particle Nature of Matter
READING MATERIAL Read About the Particle Nature of Matter PARTICLES OF MATTER DEFINITION Matter is anything that has weight and takes up space. A particle is the smallest possible unit of matter. Understanding that matter is made of tiny particles too small to be seen can help us understand the behavior and properties of matter. To better understand how the 3 states of matter work…. LET’S BREAK IT DOWN! All matter is made of particles that are too small to be seen. Everything you can see and touch is made of matter. It is all the “stuff” in the universe. Things that are not made of matter include energy, and ideas like peace and love. Matter is made up of small particles that are too small to be seen, even with a powerful microscope. They are so small that you would have to put about 100,000 particles in a line to equal the width of a human hair! Page 1 The arrangement of particles determines the state of matter. Particles are arranged and move differently in each state of matter. Solids contain particles that are tightly packed, with very little space between particles. If an object can hold its own shape and is difficult to compress, it is a solid. Liquids contain particles that are more loosely packed than solids, but still closely packed compared to gases. Particles in liquids are able to slide past each other, or flow, to take the shape of their container. Particles are even more spread apart in gases. Gases will fill any container, but if they are not in a container, they will escape into the air. -

The Positons of the Three Quarks Composing the Proton Are Illustrated
The posi1ons of the three quarks composing the proton are illustrated by the colored spheres. The surface plot illustrates the reduc1on of the vacuum ac1on density in a plane passing through the centers of the quarks. The vector field illustrates the gradient of this reduc1on. The posi1ons in space where the vacuum ac1on is maximally expelled from the interior of the proton are also illustrated by the tube-like structures, exposing the presence of flux tubes. a key point of interest is the distance at which the flux-tube formaon occurs. The animaon indicates that the transi1on to flux-tube formaon occurs when the distance of the quarks from the center of the triangle is greater than 0.5 fm. again, the diameter of the flux tubes remains approximately constant as the quarks move to large separaons. • Three quarks indicated by red, green and blue spheres (lower leb) are localized by the gluon field. • a quark-an1quark pair created from the gluon field is illustrated by the green-an1green (magenta) quark pair on the right. These quark pairs give rise to a meson cloud around the proton. hEp://www.physics.adelaide.edu.au/theory/staff/leinweber/VisualQCD/Nobel/index.html Nucl. Phys. A750, 84 (2005) 1000000 QCD mass 100000 Higgs mass 10000 1000 100 Mass (MeV) 10 1 u d s c b t GeV HOW does the rest of the proton mass arise? HOW does the rest of the proton spin (magnetic moment,…), arise? Mass from nothing Dyson-Schwinger and Lattice QCD It is known that the dynamical chiral symmetry breaking; namely, the generation of mass from nothing, does take place in QCD. -

A Young Physicist's Guide to the Higgs Boson
A Young Physicist’s Guide to the Higgs Boson Tel Aviv University Future Scientists – CERN Tour Presented by Stephen Sekula Associate Professor of Experimental Particle Physics SMU, Dallas, TX Programme ● You have a problem in your theory: (why do you need the Higgs Particle?) ● How to Make a Higgs Particle (One-at-a-Time) ● How to See a Higgs Particle (Without fooling yourself too much) ● A View from the Shadows: What are the New Questions? (An Epilogue) Stephen J. Sekula - SMU 2/44 You Have a Problem in Your Theory Credit for the ideas/example in this section goes to Prof. Daniel Stolarski (Carleton University) The Usual Explanation Usual Statement: “You need the Higgs Particle to explain mass.” 2 F=ma F=G m1 m2 /r Most of the mass of matter lies in the nucleus of the atom, and most of the mass of the nucleus arises from “binding energy” - the strength of the force that holds particles together to form nuclei imparts mass-energy to the nucleus (ala E = mc2). Corrected Statement: “You need the Higgs Particle to explain fundamental mass.” (e.g. the electron’s mass) E2=m2 c4+ p2 c2→( p=0)→ E=mc2 Stephen J. Sekula - SMU 4/44 Yes, the Higgs is important for mass, but let’s try this... ● No doubt, the Higgs particle plays a role in fundamental mass (I will come back to this point) ● But, as students who’ve been exposed to introductory physics (mechanics, electricity and magnetism) and some modern physics topics (quantum mechanics and special relativity) you are more familiar with.. -

First Determination of the Electric Charge of the Top Quark
First Determination of the Electric Charge of the Top Quark PER HANSSON arXiv:hep-ex/0702004v1 1 Feb 2007 Licentiate Thesis Stockholm, Sweden 2006 Licentiate Thesis First Determination of the Electric Charge of the Top Quark Per Hansson Particle and Astroparticle Physics, Department of Physics Royal Institute of Technology, SE-106 91 Stockholm, Sweden Stockholm, Sweden 2006 Cover illustration: View of a top quark pair event with an electron and four jets in the final state. Image by DØ Collaboration. Akademisk avhandling som med tillst˚and av Kungliga Tekniska H¨ogskolan i Stock- holm framl¨agges till offentlig granskning f¨or avl¨aggande av filosofie licentiatexamen fredagen den 24 november 2006 14.00 i sal FB54, AlbaNova Universitets Center, KTH Partikel- och Astropartikelfysik, Roslagstullsbacken 21, Stockholm. Avhandlingen f¨orsvaras p˚aengelska. ISBN 91-7178-493-4 TRITA-FYS 2006:69 ISSN 0280-316X ISRN KTH/FYS/--06:69--SE c Per Hansson, Oct 2006 Printed by Universitetsservice US AB 2006 Abstract In this thesis, the first determination of the electric charge of the top quark is presented using 370 pb−1 of data recorded by the DØ detector at the Fermilab Tevatron accelerator. tt¯ events are selected with one isolated electron or muon and at least four jets out of which two are b-tagged by reconstruction of a secondary decay vertex (SVT). The method is based on the discrimination between b- and ¯b-quark jets using a jet charge algorithm applied to SVT-tagged jets. A method to calibrate the jet charge algorithm with data is developed. A constrained kinematic fit is performed to associate the W bosons to the correct b-quark jets in the event and extract the top quark electric charge. -

The Ghost Particle 1 Ask Students If They Can Think of Some Things They Cannot Directly See but They Know Exist
Original broadcast: February 21, 2006 BEFORE WATCHING The Ghost Particle 1 Ask students if they can think of some things they cannot directly see but they know exist. Have them provide examples and reasoning for PROGRAM OVERVIEW how they know these things exist. NOVA explores the 70–year struggle so (Some examples and evidence of their existence include: [bacteria and virus- far to understand the most elusive of all es—illnesses], [energy—heat from the elementary particles, the neutrino. sun], [magnetism—effect on a com- pass], and [gravity—objects falling The program: towards Earth].) How do scientists • relates how the neutrino first came to be theorized by physicist observe and measure things that cannot be seen with the naked eye? Wolfgang Pauli in 1930. (They use instruments such as • notes the challenge of studying a particle with no electric charge. microscopes and telescopes, and • describes the first experiment that confirmed the existence of the they look at how unseen things neutrino in 1956. affect other objects.) • recounts how scientists came to believe that neutrinos—which are 2 Review the structure of an atom, produced during radioactive decay—would also be involved in including protons, neutrons, and nuclear fusion, a process suspected as the fuel source for the sun. electrons. Ask students what they know about subatomic particles, • tells how theoretician John Bahcall and chemist Ray Davis began i.e., any of the various units of mat- studying neutrinos to better understand how stars shine—Bahcall ter below the size of an atom. To created the first mathematical model predicting the sun’s solar help students better understand the neutrino production and Davis designed an experiment to measure size of some subatomic particles, solar neutrinos. -

On Particle Physics
On Particle Physics Searching for the Fundamental US ATLAS The continuing search for the basic building blocks of matter is the US ATLAS subject of Particle Physics (also called High Energy Physics). The idea of fundamental building blocks has evolved from the concept of four elements (earth, air, fire and water) of the Ancient Greeks to the nineteenth century picture of atoms as tiny “billiard balls.” The key word here is FUNDAMENTAL — objects which are simple and have no structure — they are not made of anything smaller! Our current understanding of these fundamental constituents began to fall into place around 100 years ago, when experimenters first discovered that the atom was not fundamental at all, but was itself made of smaller building blocks. Using particle probes as “microscopes,” scientists deter- mined that an atom has a dense center, or NUCLEUS, of positive charge surrounded by a dilute “cloud” of light, negatively- charged electrons. In between the nucleus and electrons, most of the atom is empty space! As the particle “microscopes” became more and more powerful, scientists found that the nucleus was composed of two types of yet smaller constituents called protons and neutrons, and that even pro- tons and neutrons are made up of smaller particles called quarks. The quarks inside the nucleus come in two varieties, called “up” or u-quark and “down” or d-quark. As far as we know, quarks and electrons really are fundamental (although experimenters continue to look for evidence to the con- trary). We know that these fundamental building blocks are small, but just how small are they? Using probes that can “see” down to very small distances inside the atom, physicists know that quarks and electrons are smaller than 10-18 (that’s 0.000 000 000 000 000 001! ) meters across. -
Proton Therapy ACKNOWLEDGEMENTS
AMERICAN BRAIN TUMOR ASSOCIATION Proton Therapy ACKNOWLEDGEMENTS ABOUT THE AMERICAN BRAIN TUMOR ASSOCIATION Founded in 1973, the American Brain Tumor Association (ABTA) was the first national nonprofit organization dedicated solely to brain tumor research. For over 40 years, the Chicago-based ABTA has been providing comprehensive resources that support the complex needs of brain tumor patients and caregivers, as well as the critical funding of research in the pursuit of breakthroughs in brain tumor diagnosis, treatment and care. To learn more about the ABTA, visit www.abta.org. We gratefully acknowledge Anita Mahajan, Director of International Development, MD Anderson Proton Therapy Center, director, Pediatric Radiation Oncology, co-section head of Pediatric and CNS Radiation Oncology, The University of Texas MD Anderson Cancer Center; Kevin S. Oh, MD, Department of Radiation Oncology, Massachusetts General Hospital; and Sridhar Nimmagadda, PhD, assistant professor of Radiology, Medicine and Oncology, Johns Hopkins University, for their review of this edition of this publication. This publication is not intended as a substitute for professional medical advice and does not provide advice on treatments or conditions for individual patients. All health and treatment decisions must be made in consultation with your physician(s), utilizing your specific medical information. Inclusion in this publication is not a recommendation of any product, treatment, physician or hospital. COPYRIGHT © 2015 ABTA REPRODUCTION WITHOUT PRIOR WRITTEN PERMISSION IS PROHIBITED AMERICAN BRAIN TUMOR ASSOCIATION Proton Therapy INTRODUCTION Brain tumors are highly variable in their treatment and prognosis. Many are benign and treated conservatively, while others are malignant and require aggressive combinations of surgery, radiation and chemotherapy. -

Properties of Baryons in the Chiral Quark Model
Properties of Baryons in the Chiral Quark Model Tommy Ohlsson Teknologie licentiatavhandling Kungliga Tekniska Hogskolan¨ Stockholm 1997 Properties of Baryons in the Chiral Quark Model Tommy Ohlsson Licentiate Dissertation Theoretical Physics Department of Physics Royal Institute of Technology Stockholm, Sweden 1997 Typeset in LATEX Akademisk avhandling f¨or teknologie licentiatexamen (TeknL) inom ¨amnesomr˚adet teoretisk fysik. Scientific thesis for the degree of Licentiate of Engineering (Lic Eng) in the subject area of Theoretical Physics. TRITA-FYS-8026 ISSN 0280-316X ISRN KTH/FYS/TEO/R--97/9--SE ISBN 91-7170-211-3 c Tommy Ohlsson 1997 Printed in Sweden by KTH H¨ogskoletryckeriet, Stockholm 1997 Properties of Baryons in the Chiral Quark Model Tommy Ohlsson Teoretisk fysik, Institutionen f¨or fysik, Kungliga Tekniska H¨ogskolan SE-100 44 Stockholm SWEDEN E-mail: [email protected] Abstract In this thesis, several properties of baryons are studied using the chiral quark model. The chiral quark model is a theory which can be used to describe low energy phenomena of baryons. In Paper 1, the chiral quark model is studied using wave functions with configuration mixing. This study is motivated by the fact that the chiral quark model cannot otherwise break the Coleman–Glashow sum-rule for the magnetic moments of the octet baryons, which is experimentally broken by about ten standard deviations. Configuration mixing with quark-diquark components is also able to reproduce the octet baryon magnetic moments very accurately. In Paper 2, the chiral quark model is used to calculate the decuplet baryon ++ magnetic moments. The values for the magnetic moments of the ∆ and Ω− are in good agreement with the experimental results. -
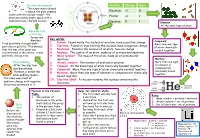
Key Words: 1. Proton: Found Inside the Nucleus of an Atom, Have a Positive Charge 2. Electron: Found in Rings Orbiting the Nucle
Nucleus development Particle Charge Mass This experiment allowed Rutherford to replace the plum pudding Electron -1 0 model with the nuclear model – the Proton +1 1 atom was mainly empty space with a small positively charged nucleus Neutron 0 1 Element All the same type of atom Alpha particle scattering Geiger and Key words: Marsden 1. Proton: Found inside the nucleus of an atom, have a positive charge Compound fired positively-charged alpha More than one type 2. Electron: Found in rings orbiting the nucleus, have a negative charge particles at gold foil. This showed of atom chemically that the mas of an atom was 3. Neutrons: Found in the nucleus of an atom, have no charge bonded together concentrated in the centre, it was 4. Nucleus: The centre of an atom, made up of protons and neutrons positively charged too 5. Mass number: The mass of the atom, made up of protons and neutrons Mixture Plum pudding 6. Atomic number: The number of protons in an atom More than one type After the electron 7. Element: All the same type of atom chemically bonded together of element or was discovered, 8. Compound: More than one type of atom chemically bonded together compound not chemically bound Thomson created the 9. Mixture: More than one type of element or compound not chemically together plum pudding model – bound together the atom was a ball of 10. Electron Shell: A ring surrounding the nucleus containing the positive charge with negative electrons electrons scattered in it Position in the Periodic Rules for electron shells: Table: 1.