Association of Changes in Norepinephrine and Serotonin Transporter Expression with the Long-Term Behavioral Effects of Antidepressant Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Monoamine Oxydases Et Athérosclérose : Signalisation Mitogène Et Études in Vivo
UNIVERSITE TOULOUSE III - PAUL SABATIER Sciences THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE TOULOUSE III Discipline : Innovation Pharmacologique Présentée et soutenue par : Christelle Coatrieux le 08 octobre 2007 Monoamine oxydases et athérosclérose : signalisation mitogène et études in vivo Jury Monsieur Luc Rochette Rapporteur Professeur, Université de Bourgogne, Dijon Monsieur Ramaroson Andriantsitohaina Rapporteur Directeur de Recherche, INSERM, Angers Monsieur Philippe Valet Président Professeur, Université Paul Sabatier, Toulouse III Madame Nathalie Augé Examinateur Chargé de Recherche, INSERM Monsieur Angelo Parini Directeur de Thèse Professeur, Université Paul Sabatier, Toulouse III INSERM, U858, équipes 6/10, Institut Louis Bugnard, CHU Rangueil, Toulouse Résumé Les espèces réactives de l’oxygène (EROs) sont impliquées dans l’activation de nombreuses voies de signalisation cellulaires, conduisant à différentes réponses comme la prolifération. Les EROs, à cause du stress oxydant qu’elles génèrent, sont impliquées dans de nombreuses pathologies, notamment l’athérosclérose. Les monoamine oxydases (MAOs) sont deux flavoenzymes responsables de la dégradation des catécholamines et des amines biogènes comme la sérotonine ; elles sont une source importante d’EROs. Il a été montré qu’elles peuvent être impliquées dans la prolifération cellulaire ou l’apoptose du fait du stress oxydant qu’elles génèrent. Ce travail de thèse a montré que la MAO-A, en dégradant son substrat (sérotonine ou tyramine), active une voie de signalisation mitogène particulière : la voie métalloprotéase- 2/sphingolipides (MMP2/sphingolipides), et contribue à la prolifération de cellules musculaire lisses vasculaires induite par ces monoamines. De plus, une étude complémentaire a confirmé l’importance des EROs comme stimulus mitogène (utilisation de peroxyde d’hydrogène exogène), et a décrit plus spécifiquement les étapes en amont de l’activation de MMP2, ainsi que l’activation par la MMP2 de la sphingomyélinase neutre (première enzyme de la cascade des sphingolipides). -

Does the Antidepressant-Like Effect of Mirtazapine and Venlafaxine Differ Between Male and Female Rats?
ORIGINAL ARTICLE Volume 43, Issue 1, January-February 2020 doi: 10.17711/SM.0185-3325.2020.002 Does the antidepressant-like effect of mirtazapine and venlafaxine differ between male and female rats? Adriana Álvarez Silva,1 Alonso Fernández-Guasti1 1 Departamento de Farmacobiología, ABSTRACT Centro de Investigación y de Estu- dios Avanzados del Instituto Politécni- Depression is a global health problem with nearly 350 million people affected, mainly women. co Nacional, Ciudad de México, Introduction. México. However, nowadays a rising amount of men are being diagnosed. This makes necessary the screening of new treatment options that are effective in women as well as in men. Objective. To analyze if the administration of Correspondence: mirtazapine and venlafaxine to male and female rats shows a sex-related antidepressant-like effect, and the Alonso Fernández-Guasti Laboratorio de Farmacología possible associated neurochemical mechanisms. Method. Mirtazapine (40 mg/kg) or venlafaxine (60 mg/kg) Conductual, Centro de Investigación were administered subchronically to young adult male and female (ovariectomized and steroid-primed) rats, y de Estudios Avanzados del Instituto and their antidepressant-like effects were evaluated using the forced swim test (FST). The active behaviors, Politécnico Nacional. swimming and climbing, were also analyzed. a) mirtazapine and venlafaxine reduced immobility Calzada de los Tenorios 235, Results. Col Granjas Coapa, in the FST in males and females; b) both antidepressants increased climbing and swimming in male rats; Alcaldía Tlalpan, c) in female rats, mirtazapine and venlafaxine only increased swimming. Discussion and conclusion. In 14330, Ciudad de México, México. males, the effects of mirtazapine and venlafaxine seem to be produced by the activation of the serotonergic Phone: +52 (55) 5483 - 2848 and noradrenergic systems. -

Antinociceptive Effects of Monoamine Reuptake Inhibitors in Assays of Pain-Stimulated and Pain-Depressed Behaviors
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2012 Antinociceptive Effects of Monoamine Reuptake Inhibitors in Assays of Pain-Stimulated and Pain-Depressed Behaviors Marisa Rosenberg Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Medical Pharmacology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2715 This Thesis is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. ANTINOCICEPTIVE EFFECTS OF MONOAMINE REUPTAKE INHIBITORS IN ASSAYS OF PAIN-STIMULATED AND PAIN-DEPRESSED BEHAVIOR A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science at Virginia Commonwealth University By Marisa B. Rosenberg Bachelor of Science, Temple University, 2008 Advisor: Sidney Stevens Negus, Ph.D. Professor, Department of Pharmacology/Toxicology Virginia Commonwealth University Richmond, VA May, 2012 Acknowledgement First and foremost, I’d like to thank my advisor Dr. Steven Negus, whose unwavering support, guidance and patience throughout my graduate career has helped me become the scientist I am today. His dedication to education, learning and the scientific process has instilled in me a quest for knowledge that I will continue to pursue in life. His thoroughness, attention to detail and understanding of pharmacology has been exemplary to a young person like me just starting out in the field of science. I’d also like to thank all of my committee members (Drs. -

Product List March 2019 - Page 1 of 53
Wessex has been sourcing and supplying active substances to medicine manufacturers since its incorporation in 1994. We supply from known, trusted partners working to full cGMP and with full regulatory support. Please contact us for details of the following products. Product CAS No. ( R)-2-Methyl-CBS-oxazaborolidine 112022-83-0 (-) (1R) Menthyl Chloroformate 14602-86-9 (+)-Sotalol Hydrochloride 959-24-0 (2R)-2-[(4-Ethyl-2, 3-dioxopiperazinyl) carbonylamino]-2-phenylacetic 63422-71-9 acid (2R)-2-[(4-Ethyl-2-3-dioxopiperazinyl) carbonylamino]-2-(4- 62893-24-7 hydroxyphenyl) acetic acid (r)-(+)-α-Lipoic Acid 1200-22-2 (S)-1-(2-Chloroacetyl) pyrrolidine-2-carbonitrile 207557-35-5 1,1'-Carbonyl diimidazole 530-62-1 1,3-Cyclohexanedione 504-02-9 1-[2-amino-1-(4-methoxyphenyl) ethyl] cyclohexanol acetate 839705-03-2 1-[2-Amino-1-(4-methoxyphenyl) ethyl] cyclohexanol Hydrochloride 130198-05-9 1-[Cyano-(4-methoxyphenyl) methyl] cyclohexanol 93413-76-4 1-Chloroethyl-4-nitrophenyl carbonate 101623-69-2 2-(2-Aminothiazol-4-yl) acetic acid Hydrochloride 66659-20-9 2-(4-Nitrophenyl)ethanamine Hydrochloride 29968-78-3 2,4 Dichlorobenzyl Alcohol (2,4 DCBA) 1777-82-8 2,6-Dichlorophenol 87-65-0 2.6 Diamino Pyridine 136-40-3 2-Aminoheptane Sulfate 6411-75-2 2-Ethylhexanoyl Chloride 760-67-8 2-Ethylhexyl Chloroformate 24468-13-1 2-Isopropyl-4-(N-methylaminomethyl) thiazole Hydrochloride 908591-25-3 4,4,4-Trifluoro-1-(4-methylphenyl)-1,3-butane dione 720-94-5 4,5,6,7-Tetrahydrothieno[3,2,c] pyridine Hydrochloride 28783-41-7 4-Chloro-N-methyl-piperidine 5570-77-4 -

Slang Terms and Code Words: a Reference for Law Enforcement
UNCLASSIFIED Slang Terms and Code Words: A Reference for Law DEA Enforcement Personnel Intelligence DEA-HOU-DIR-022-18 July 2018 ReportBrief 1 UNCLASSIFIED UNCLASSIFIED DEA Intelligence Report Executive Summary This Drug Enforcement Administration (DEA) Intelligence Report contains new and updated information on slang terms and code words from a variety of law enforcement and open sources, and serves as an updated version to the product entitled “Drug Slang Code Words” published by the DEA in May 2017. It is designed as a ready reference for law enforcement personnel who are confronted with hundreds of slang terms and code words used to identify a wide variety of controlled substances, designer drugs, synthetic compounds, measurements, locations, weapons, and other miscellaneous terms relevant to the drug trade. Although every effort was made to ensure the accuracy and completeness of the information presented, due to the dynamics of the ever-changing drug scene, subsequent additions, deletions, and corrections are inevitable. Future addendums and updates to this report will attempt to capture changed terminology to the furthest extent possible. This compendium of slang terms and code words is alphabetically ordered, with new additions presented in italic text, and identifies drugs and drug categories in English and foreign language derivations. Drug Slang Terms and Code Wordsa Acetaminophen and Oxycodone Combination (Percocet®) 512s; Bananas; Blue; Blue Dynamite; Blueberries; Buttons; Ercs; Greenies; Hillbilly Heroin; Kickers; M-30s; -

Cipramil® 20 Mg Film-Coated Tablets
NEW ZEALAND DATA SHEET 1 NAME OF THE MEDICINE Cipramil® 20 mg Film-coated Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Cipramil 20 mg Film-coated tablets contain 24.98 mg citalopram hydrobromide, corresponding to 20 mg citalopram base. Excipients with known effect: lactose For the full list of excipients, see Section 6.1 List of excipients. 3 PHARMACEUTICAL FORM Cipramil tablets are oval, white, film-coated tablets, 8 mm × 5.5 mm, marked “C” and “N” symmetrically around the score-line. 4 CLINICAL PARTICULARS 4.1 Therapeutic indications Treatment of depressive illness in the initial phase and as maintenance against potential relapse/ recurrence. 4.2 Dose and method of administration The dose may be taken in the morning or evening without regard for food. As the treatment result in general can be evaluated only after 2-3 weeks’ treatment, a possible dose increase in increments of 10 mg should take place with intervals of 2-3 weeks. Adults Cipramil should be administered as a single oral dose of 20 mg daily. Dependent on individual patient response and severity of depression the dose may be increased to a maximum of 40 mg daily. The maximum daily dose should not be exceeded as doses above 40mg/day are associated with an increased risk of QT prolongation. Elderly patients The starting dose is 10mg/day. The dose can be increased by 10mg to a maximum of 20mg/day. Use in children and adolescents (under 18 years of age) Safety and efficacy have not been established in this population. Consequently, citalopram should not be used in children and adolescents under 18 years of age (see Section 4.4 Special warnings and precautions or use). -

Targeting the Tryptophan Hydroxylase 2 Gene for Functional Analysis in Mice and Serotonergic Differentiation of Embryonic Stem Cells
TARGETING THE TRYPTOPHAN HYDROXYLASE 2 GENE FOR FUNCTIONAL ANALYSIS IN MICE AND SEROTONERGIC DIFFERENTIATION OF EMBRYONIC STEM CELLS Inaugural-Dissertation to obtain the academic degree Doctor rerum naturalium (Dr. rer. nat.) submitted to the Department of Biology, Chemistry and Pharmacy of Freie Universität Berlin by Dana Kikic, M.Sc. in Molecular biology and Physiology from Nis June, 2009 The doctorate studies were performed in the research group of Prof. Michael Bader Molecular Biology of Peptide Hormones at Max-Delbrück-Center for Molecular Medicine in Berlin, Buch Mai 2005 - September 2008. 1st Reviewer: Prof. Michael Bader 2nd Reviewer: Prof. Udo Heinemann date of defence: 13. August 2009 ACKNOWLEDGMENTS Herewith, I would like to acknowledge the persons who made this thesis possible and without whom my initiation in the world of basic science research would not have the spin it has now, neither would my scientific illiteracy get the chance to eradicate. I am expressing my very personal gratitude and recognition to: Prof. Michael Bader, for an inexhaustible guidance in all the matters arising during the course of scientific work, for an instinct in defining and following the intellectual challenge and for letting me following my own, for necessary financial support, for defining the borders of reasonable and unreasonable, for an invaluable time and patience, and an amazing efficiency in supporting, motivating, reading, correcting and shaping my scientific language during the last four years. Prof. Harald Saumweber and Prof. Udo Heinemann, for taking over the academic supervision of the thesis, and for breathing in it a life outside the laboratory walls and their personal signature. -

A Synbiotic Mixture Augmented the Efficacy of Doxepin, Venlafaxine
A synbiotic mixture augmented the efficacy of doxepin, venlafaxine, and fluvoxamine in mice model of depression Azadeh Mesripour1, Andiya Meshkati1, Valiolah Hajhashemi2 1Department of Pharmacology and Toxicology, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, IRAN. 2‐ Isfahan Pharmaceutical Sciences Research Center, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, IRAN. ABSTRACT Objective: Currently available antidepressant drugs have notable downsides; in addition to their side effects and slow onset of action their moderate efficacy in some individuals, may influence compliance. Previous literature has shown that probiotics may have antidepressant effects. Introducing complementary medicineproof in order to augment the efficacy of therapeutic doses of antidepressant drugs seems to be very important. Therefore the effect of adding a synbiotic cocktail in drinking water was assessed in mice model of despair following administrating three antidepressant drugs belonging to different classes. Methods: The marble burring test (MBT), and forced swimming test (FST) were used as animal model of obsessive behavior and despair. The synbiotic cocktail was administered in mice drinking water (6.25×106 CFU) for 14 days and the tests were performed on the days 7 and 14 thirty minutes after injecting the lowest dose of doxepin (1 mg/kg), venlafaxine (15 mg/kg), and fluvoxamine (15 mg/kg). Results: After 7 days of the synbiotic ingestion immobility time decreased in FST for doxepin (92 sec ± 5.5) and venlafaxine (17.3 sec ± 2.5) compared to their control group (drinking water) but fluvoxamine could decrease immobility time after 14 days of ingesting the synbiotic (70 sec ± 7.5). -

TAYSIDE PRESCRIBER Issue No
TAYSIDE PRESCRIBER Issue No. 122 – May 2012 Produced by the NS Tayside Medicines Governance Unit in conjunction with Mental Health Citalopram & escitalopram:QT interval prolongation New maximum daily dose restrictions, contraindications, and warnings Information has been issued via Drug Safety Update, Volume 5, Issue 5, December 2011 and ‘Dear Healthcare Professional Letters’ for both citalopram and escitalopram regarding new restrictions on the maximum daily doses, contraindications, and warnings. This is as a result of an assessment of a QT study that revealed dose-dependent increase in the QT interval observed with ECG monitoring for both citalopram and escitalopram. Maximum licensed daily doses for citalopram and escitalopram Adults Adults > 65 years Adults with hepatic impairment Citalopram 40 mg 20 mg 20 mg Escitalopram (non-formulary) 20 mg 10 mg 10mg The guidance in NHS Tayside is: ⇒ to review all patients on high dose* citalopram or escitalopram with aim of reducing to new maximum licensed doses ( * above new maximum licensed daily doses as stated in the table above) ⇒ not to prescribe citalopram or escitalopram with other medication known to prolong the QT interval ⇒ not to prescribe citalopram and escitalopram in patients with known QT prolongation or congenital long QT syndrome ⇒ to consider alternative antidepressant in patients with cardiac disease ( e.g. patients with significant bradycardia; recent myocardial infarction or decompensated heart failure) See flow diagram on page 3 for further guidance and table below on medicines known to prolong the QT interval. Medicines known to increase plasma levels of citalopram or escitalopram, e.g. omeprazole & some antivirals may require dose reduction of citalopram or escitalopram and should be used with caution. -
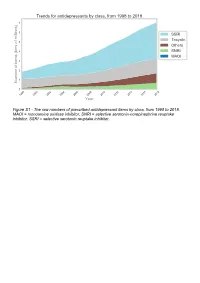
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

Carbon-I I-D-Threo-Methylphenidate Binding to Dopamine Transporter in Baboon Brain
Carbon-i i-d-threo-Methylphenidate Binding to Dopamine Transporter in Baboon Brain Yu-Shin Ding, Joanna S. Fowler, Nora D. Volkow, Jean Logan, S. John Gatley and Yuichi Sugano Brookhaven National Laboratory, Upton, New York; and Department of Psychiatry, SUNY Stony Brook@Stony Brook@NY children (1). MP is also used to treat narcolepsy (2). The The more active d-enantiomer of methyiphenidate (dI-threo psychostimulant properties of MP have been linked to its methyl-2-phenyl-2-(2-piperidyl)acetate, Ritalin)was labeled with binding to a site on the dopamine transporter, resulting in lic (t1,@:20.4 mm) to characterize its binding, examine its spec inhibition of dopamine reuptake and enhanced levels of ificftyfor the dopamine transporter and evaluate it as a radio synaptic dopamine. tracer forthe presynapticdopaminergicneuron. Methods PET We have developed a rapid synthesis of [11C]dl-threo studies were canied out inthe baboon. The pharmacokinetics of methylphenidate ([“C]MP)to examine its pharmacokinet r1c]d-th@O-msth@ha@idate @f'1C]d-thmo-MP)weremeasured ics and pharmacological profile in vivo and to evaluate its and compared with r1cY-th@o-MP and with fts racemate ff@1C]fl-thmo-meth@1phenidate,r1c]MP). Nonradioact,ve meth suitability as a radiotracer for the presynaptic dopaminergic ylphenidate was used to assess the reveralbilityand saturability neuron (3,4). These first PET studies of MP in the baboon of the binding. GBR 12909, 3@3-(4-iodophenyI)tropane-2-car and human brain demonstrated the saturable [1‘C]MP boxylic acid methyl ester (fi-Cfl), tomoxetine and citalopram binding to the dopamine transporter in the baboon brain were used to assess the binding specificity. -

Examination of Antimicrobial Activity of Selected Non-Antibiotic Medicinal Preparations
Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 69 No. 6 pp.1368ñ1371, 2012 ISSN 0001-6837 Polish Pharmaceutical Society EXAMINATION OF ANTIMICROBIAL ACTIVITY OF SELECTED NON-ANTIBIOTIC MEDICINAL PREPARATIONS HANNA KRUSZEWSKA1*, TOMASZ ZAR BA1 and STEFAN TYSKI1,2 1National Medicines Institute, Department of Antibiotics and Microbiology, 30-34 Che≥mska St., 00-725 Warszawa, Poland 2 Medical University of Warsaw, Department of Pharmaceutical Microbiology, 3 Oczki St., 02-007 Warszawa, Poland Abstract: The aim of this study was to detect and characterize the antimicrobial activity of non-antibiotic drugs, selected from the pharmaceutical products analyzed during the state control performed in National Medicines Institute, Warszawa, Poland. In 2010, over 90 pharmaceutical preparations have been randomly chosen from different groups of drugs. The surveillance study was performed on standard ATCC microbial strains used for drug control: S. aureus, E. coli, P. aeruginosa and C. albicans. It was shown that the drugs listed below inhib- ited growth of at least one of the examined strains: Arketis 20 mg tab. (paroxetine), Buvasodil 150 mg tab. (buflomedile), Halidor 100 mg tab. (bencyclane), Hydroxyzinum espefa 25 mg tab. (hydroxyzine), Norifaz 35 mg tab. (risedronate), Strattera 60 mg cap. (atomoxetine), Tamiflu 75 mg tab. (oseltamivir), Valpro-ratiopharm Chrono 300 mg tab. with longer dissolution (valproate), Vetminth oral paste 24 g+3 g/100 mL (niclozamide, oxybendazol). Strattera cap. showed broad activity spectrum. It inhibited growth of