Hemodialysis Drug Removal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparison of Early Intensive Methotrexate/Mercaptopurine With
Leukemia (2001) 15, 1038–1045 2001 Nature Publishing Group All rights reserved 0887-6924/01 $15.00 www.nature.com/leu A comparison of early intensive methotrexate/mercaptopurine with early intensive alternating combination chemotherapy for high-risk B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group phase III randomized trial SJ Lauer1, JJ Shuster2, DH Mahoney Jr3, N Winick4, S Toledano5, L Munoz5, G Kiefer6, JD Pullen7, CP Steuber3 and BM Camitta6 1Emory University School of Medicine, Atlanta, GA; 2Pediatric Oncology Group Statistical Office and Department of Statistics, University of Florida, Gainesville, FL; 3Texas Children’s Cancer Center, Baylor College of Medicine, Houston, TX; 4University of Texas Southwestern Medical Center, Dallas, TX; 5University Miami School of Medicine, Miami, FL; 6Midwest Children’s Cancer Center, Milwaukee, WI; and 7University of Mississippi Medical Center Children’s Hospital, Jackson, MS, USA A prospective, randomized multicenter study was performed to to their risk for relapse and treatment strategies designed to evaluate the relative efficacy of two different concepts for early improve event-free survival (EFS). intensive therapy in a randomized trial of children with B-pre- cursor acute lymphoblastic leukemia (ALL) at high risk (HR) for It is believed that the leading causes of relapse in children relapse. Four hundred and ninety eligible children with HR-ALL with higher risk ALL (HR-ALL) are inadequate cell kill and were randomized on the Pediatric Oncology Group (POG) 9006 emergence of drug-resistant clones. Clinical trials using early phase III trial between 7 January 1991 and 12 January 1994. intensive myelosuppressive combination chemotherapy as After prednisone (PDN), vincristine (VCR), asparaginase (ASP) post-induction consolidation were designed to maximize cell and daunorubicin (DNR) induction, 470 patients received either 2 kill and address drug resistance. -

Incidence of Differentiation Syndrome Associated with Treatment
Journal of Clinical Medicine Review Incidence of Differentiation Syndrome Associated with Treatment Regimens in Acute Myeloid Leukemia: A Systematic Review of the Literature Lucia Gasparovic 1, Stefan Weiler 1,2, Lukas Higi 1 and Andrea M. Burden 1,* 1 Institute of Pharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zurich, 8093 Zurich, Switzerland; [email protected] (L.G.); [email protected] (S.W.); [email protected] (L.H.) 2 National Poisons Information Centre, Tox Info Suisse, Associated Institute of the University of Zurich, 8032 Zurich, Switzerland * Correspondence: [email protected]; Tel.: +41-76-685-22-56 Received: 30 August 2020; Accepted: 14 October 2020; Published: 18 October 2020 Abstract: Differentiation syndrome (DS) is a potentially fatal adverse drug reaction caused by the so-called differentiating agents such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), used for remission induction in the treatment of the M3 subtype of acute myeloid leukemia (AML), acute promyelocytic leukemia (APL). However, recent DS reports in trials of isocitrate dehydrogenase (IDH)-inhibitor drugs in patients with IDH-mutated AML have raised concerns. Given the limited knowledge of the incidence of DS with differentiating agents, we conducted a systematic literature review of clinical trials with reports of DS to provide a comprehensive overview of the medications associated with DS. In particular, we focused on the incidence of DS reported among the IDH-inhibitors, compared to existing ATRA and ATO therapies. We identified 44 published articles, encompassing 39 clinical trials, including 6949 patients. Overall, the cumulative incidence of DS across all treatment regimens was 17.7%. -

Daunorubicin Hydrochloride Injection Hikma Pharmaceuticals USA Inc
DAUNORUBICIN HYDROCHLORIDE- daunorubicin hydrochloride injection Hikma Pharmaceuticals USA Inc. ---------- DAUNORUBICIN HYDROCHLORIDE INJECTION Rx ONLY WARNINGS 1.Daunorubicin Hydrochloride Injection must be given into a rapidly flowing intravenous infusion. It must never be given by the intramuscular or subcutaneous route. Severe local tissue necrosis will occur if there is extravasation during administration. 2.Myocardial toxicity manifested in its most severe form by potentially fatal congestive heart failure may occur either during therapy or months to years after termination of therapy. The incidence of myocardial toxicity increases after a total cumulative dose exceeding 400 to 550 mg/m2 in adults, 300 mg/m2 in children more than 2 years of age, or 10 mg/kg in children less than 2 years of age. 3.Severe myelosuppression occurs when used in therapeutic doses; this may lead to infection or hemorrhage. 4.It is recommended that daunorubicin hydrochloride be administered only by physicians who are experienced in leukemia chemotherapy and in facilities with laboratory and supportive resources adequate to monitor drug tolerance and protect and maintain a patient compromised by drug toxicity. The physician and institution must be capable of responding rapidly and completely to severe hemorrhagic conditions and/or overwhelming infection. 5.Dosage should be reduced in patients with impaired hepatic or renal function. DESCRIPTION Daunorubicin hydrochloride is the hydrochloride salt of an anthracycline cytotoxic antibiotic produced by a strain of Streptomyces coeruleorubidus. It is provided as a deep red sterile liquid in vials for intravenous administration only. Each mL contains 5 mg daunorubicin (equivalent to 5.34 mg of daunorubicin hydrochloride), 9 mg sodium chloride; sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection, q.s. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Australian Public Assessment Report for Pegaspargase
Australian Public Assessment Report for pegaspargase Proprietary Product Name: Oncaspar Sponsor: Baxalta Australia September 2018 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) • The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health and is responsible for regulating medicines and medical devices. • The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance) when necessary. • The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. • The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. • To report a problem with a medicine or medical device, please see the information on the TGA website < https://www.tga.gov.au>. About AusPARs • An Australian Public Assessment Report (AusPAR) provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission. • AusPARs are prepared and published by the TGA. • An AusPAR is prepared for submissions that relate to new chemical entities, generic medicines, major variations and extensions of indications. • An AusPAR is a static document; it provides information that relates to a submission at a particular point in time. • A new AusPAR will be developed to reflect changes to indications and/or major variations to a prescription medicine subject to evaluation by the TGA. -
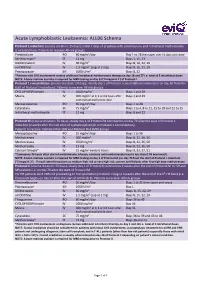
Acute Lymphoblastic Leukaemia: ALL06 Schema
Acute Lymphoblastic Leukaemia: ALL06 Schema Protocol 1 induction: (course duration: 35 days); initial 7 days of prephase with prednisolone and intrathecal methotrexate is included here. Patients to receive: All risk groups Prednisolone PO 60 mg/m2/day Day 1 to 28 then taper over 10 days and cease Methotrexate* IT 12 mg Days 1, 15, 33 DAUNOrubicin IV 30 mg/m2 Days 8, 16, 22, 29 vinCRISTine IV 1.5 mg/m2 (cap at 2 mg) Days 8, 16, 22, 29 Pegaspargase IM 1000 U/m2 Days 8, 22 *Patients with CNS involvement receive additional intrathecal methotrexate therapy on day 18 and 27 i.e. total of 5 intrathecal doses NOTE: A bone marrow aspirate is required for MRD testing on day 33 ('Timepoint 1') of Protocol I Protocol 1 consolidation: (course duration: 29 days); ideally day 1 of Protocol I consolidation commences on day 36 from the start of Protocol 1 induction ). Patients to receive: All risk groups. CYCLOPHOSPHamide IV 1000 mg/m2 Days 1 and 29 Mesna IV 400 mg/m2 at 0, 4 and 8 hours after Days 1 and 29 each cyclophosphamide dose Mercaptopurine PO 60 mg/m2/day Days 1 to 28 Cytarabine SC 75 mg/m2 Days 1 to 4, 8 to 11, 15 to 18 and 22 to 25 Intrathecal methotrexate IT 12 mg Days 8 and 22 Protocol M: (course duration: 56 days); ideally day 1 of Protocol M commences on day 79 after the start of Protocol 1 induction (2 weeks after the last dose of cyclophosphamide in Protocol 1 consolidation). -

Emetogenic Potential of Antineoplastic Agents
EMETOGENIC POTENTIAL OF ANTINEOPLASTIC AGENTS High Risk (>90% frequency without antiemetics) • AC combination: Doxorubicin or Epirubicin (Ellence) ϩ • Doxorubicin IV: >60mg/m 2 Cyclophosphamide (Cytoxan) IV • Epirubicin (Ellence) IV: >90mg/m 2 • Altretamine (HMM, Hexalen) oral • Ifosfamide (Ifex) IV: Ն2g/m 2 per dose • Carmustine (BCNU, BiCNU) IV: Ͼ250mg/m 2 • Mechlorethamine (Mustargen) IV • Cisplatin (CDDP) IV • Procarbazine (Matulane) oral • Cyclophosphamide (CTX, Cytoxan) IV: Ͼ1,500mg/m 2 • Streptozocin (Zanosar) IV • Dacarbazine (DTIC, DTIC-Dome) IV Moderate Risk (30–90% frequency without antiemetics) 2 2 • Aldesleukin (IL-2, Proleukin) IV: Ͼ12–15 million IU/m • Doxorubicin IV: Յ60mg/m 2 2 • Amifostine (Ethyol) IV: Ͼ300mg/m • Epirubicin (Ellence) IV: Յ90mg/m • Arsenic trioxide (As2 O3 , Trisenox) IV • Estramustine (Emcyt) oral • Azacitidine (Vidaza) IV • Etoposide (VP-16) oral • Bendamustine (Treanda) IV • Idarubicin (Idamycin) IV • Busulfan (Busulfex) IV ; oral: Ͼ4mg/day • Ifosfamide (Ifex) IV: Ͻ2g/m 2 • Carboplatin IV • Interferon alpha (IFN-alfa, Intron A) IV: Ն10 million IU/m 2 • Carmustine (BCNU, BiCNU) IV: Յ250mg/m 2 • Irinotecan (CPT-11, Camptosar) IV • Clofarabine (Clolar) IV • Lomustine (CCNU, CeeNU) oral • Cyclophosphamide (CTX, Cytoxan) IV: Յ1,500mg/m 2 • Melphalan (L-PAM, Alkeran) IV • Cyclophosphamide (CTX) oral Ն100mg/m 2 /day • Methotrexate (MTX) IV: Ն250mg/m 2 • Cytarabine (ARA-C) IV: Ͼ200mg/m 2 • Oxaliplatin (Eloxatin) IV • Dactinomycin (Cosmegen) IV • Temozolomide (Temodar) IV; oral Ͼ75mg/m 2 /day • Daunorubicin -

Original New Drug Applications
New Drugs Reviewed by CPG January 23, 2017 (Original New Drug Applications: FDA) Generic Name Trade Name Indication(s) CPG Action Immune globulin Cuvitru Biologic and In accordance with subcutaneous Immunological the SCA [human] 20% solution Agents/Immune Globulins/ Immune Globulin (Human) subcutaneous. Indicated as replacement for primary humoral immunodeficiency in adult and pediatric patients age two and older. eteplirsen Exondys 51 Central Nervous System In accordance with Agents/Antisense the SCA Oligonucleotides. Treatment of patients who have a confirmed mutation of the dystrophin gene amenable to exon 51 skipping. First drug approved for reatment of patients with Duchenne muscular dystrophy. levonorgestrel- Kyleena Endocrine and May prescribe releasing intrauterine Metabolic Agents/sex system 19.5 mg homones/contraceptive Hormones. Prevention of pregnancy for up to five years. **Phentermine Lomaira Central Nervous System In accordance with hydrochloride 8 mg Agents/ the SCA anorexiants/sympathomi metic anorexiants. Short term use weight reduction in adults with **(new formulation at an initial BMI of 30 or lower dosage) more or 27 with at least one weight-related condition. 1 New Drugs January 2017 canaglifozin/ Invokamet XR Endocrine and May prescribe metformin HCL Metabolic Agents/ extended-release antidiabetic agents/antidiabetic combination products. Treatment of adults with type 2 diabetes as an adjunct to diet and exercise. Adalimumab-atto Amjevita Biologic and In accordance with Immunological Agents/ the SCA Immunologic Agents/Immunomodulat ors/Tumor necrosis Factor-Alpha Blockers. Indicated for treatment of adults with: rheumatoid arthritis, psoriatic arthritis,ankylosing spondylitis, Crohn disease, ulcerative colitis, and plaque psoriasis. Also indicated for juvenile idiopathic arthritis in pts age 4 years and older. -

Oncaspar, INN-Pegaspargase
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Oncaspar 750 U/ml solution for injection/infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml of solution contains 750 Units (U)** of pegaspargase*. One vial of 5 ml solution contains 3,750 Units. * The active substance is a covalent conjugate of Escherichia coli-derived L-asparaginase with monomethoxypolyethylene glycol **One unit is defined as the quantity of enzyme required to liberate 1 µmol ammonia per minute at pH 7.3 and 37°C The potency of this medicinal product should not be compared to the one of another pegylated or non-pegylated protein of the same therapeutic class. For more information, see section 5.1. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection/infusion. Clear, colourless solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Oncaspar is indicated as a component of antineoplastic combination therapy in acute lymphoblastic leukaemia (ALL) in paediatric patients from birth to 18 years, and adult patients. 4.2 Posology and method of administration Oncaspar should be prescribed and administered by physicians and/or health care personnel experienced in the use of antineoplastic products. It should only be given in a hospital setting where appropriate resuscitation equipment is available. Patients should be closely monitored for any adverse reactions throughout the administration period (see section 4.4). Posology Oncaspar is usually administered as part of combination chemotherapy protocols with other antineoplastic agents (see also section 4.5). Paediatric patients and adults ≤21 years The recommended dose in patients with a body surface area (BSA) ≥0.6 m2 and who are ≤21 years of age is 2,500 U of pegaspargase (equivalent to 3.3 ml Oncaspar)/m2 body surface area every 14 days. -

Benefit of Intermediate-Dose Cytarabine-Containing Induction In
Letters to the Editor ter RFS and EFS rates and showed a marked tendency to Benefit of intermediate-dose cytarabine-containing improve the OS of patients with CEBPAdm in both uni- induction in molecular subgroups of acute myeloid variate and multivariable analyses, as shown in Online leukemia Supplementary Table S2. Five-year RFS, EFS, and OS rates were 85%, 81%, and 88% in the intermediate-dose com- The outcome of acute myeloid leukemia (AML) is pared with 56%, 56%, and 68% in the conventional- affected by disease characteristics as well as treatment dose group, respectively (Figure 1). In total, 13 of 75 1-3 regimens. In the CALGB8525 trial, patients with core (17%) patients with CEBPAdm AML underwent allo- binding factor (CBF)-positive leukemia benefited from geneic transplantation in CR1, including five of 32 (16%) 4 consolidation with a high dose of cytarabine. More in the conventional-dose group and eight of 43 (19%) in 2 recently, high-dose daunorubicin (60-90 mg/m ) has the intermediate-dose group. To analyze results in the 5,6 become widely used. High-dose daunorubicin confers a absence of any possible contributory effect of transplan- favorable prognosis for patients with NPM1 muta- tation, patients were censored at the time of transplanta- 1,7,8 tions. tion in CR1. Patients with CEBPAdm AML exposed to Higher-dose cytarabine was also introduced into AML intermediate-dose cytarabine achieved an increase in 5- 3,9 induction therapy. Recently, we investigated the role of year RFS, censored at the date of transplantation, from intermediate-dose cytarabine in induction therapy of 56% to 83% (hazard ratio [HR], 0.313; 95% confidence AML and found that the introduction of intermediate- interval [95% CI]: 0.119-0.824; Wald P=0.019) (Online dose cytarabine, combined with daunorubicin and omac- Supplementary Figure S3). -

Prescribing Information | VELCADE® (Bortezomib)
HIGHLIGHTS OF PRESCRIBING INFORMATION Hypotension: Use caution when treating patients taking These highlights do not include all the information needed to antihypertensives, with a history of syncope, or with dehydration. use VELCADE safely and effectively. See full prescribing (5.2) information for VELCADE. Cardiac Toxicity: Worsening of and development of cardiac VELCADE® (bortezomib) for injection, for subcutaneous or failure has occurred. Closely monitor patients with existing heart intravenous use disease or risk factors for heart disease. (5.3) Initial U.S. Approval: 2003 Pulmonary Toxicity: Acute respiratory syndromes have ------------------------RECENT MAJOR CHANGES-------------------------- occurred. Monitor closely for new or worsening symptoms and consider interrupting VELCADE therapy. (5.4) Warnings and Precautions, Posterior Reversible Encephalopathy Syndrome: Consider MRI Thrombotic Microangiopathy (5.10) 04/2019 imaging for onset of visual or neurological symptoms; ------------------------INDICATIONS AND USAGE-------------------------- discontinue VELCADE if suspected. (5.5) VELCADE is a proteasome inhibitor indicated for: Gastrointestinal Toxicity: Nausea, diarrhea, constipation, and treatment of adult patients with multiple myeloma (1.1) vomiting may require use of antiemetic and antidiarrheal medications or fluid replacement. (5.6) treatment of adult patients with mantle cell lymphoma (1.2) Thrombocytopenia and Neutropenia: Monitor complete blood ----------------------DOSAGE AND ADMINISTRATION--------------------- -

SUPPLEMENTARY FILE Cellular Fitnessphenotype of Cancer Target
SUPPLEMENTARY FILE Cellular FitnessPhenotype of Cancer Target Genes in Cancer Therapeutics Bijesh George, P. Mukundan Pillai, Aswathy Mary Paul, Revikumar Amjesh, Kim Leitzel, Suhail M. Ali, Oleta Sandiford, Allan Lipton, Pranela Rameshwar, Gabriel N. Hortobagyi, Madhavan Radhakrishna Pillai, and Rakesh Kumar Supplementary Figures Supplementary Figure S1: Fitness-dependency of cellular targets of approved oncology drugs in cancer cell lines. A, Distribution of alterations in the cellular fitness in esophageal cancer cell lines upon selective knockdown of FSPS test gene. The values are plotted as boxplots with positive and negative changes in the cellular fitness for each cell line, represented by a single dot. B, Effect of knocking down of indicated targets in Head and Neck carcinoma cell lines. C, Distribution of 47 cancer targets of FDA-approved drugs, including, a subset of its 15 priority therapeutic targets in across cancer- types for which drugs targeting these cellular targets are approved. D, Distribution of 47 fitness targets across 19 cancer types for which drugs targeting these molecules are approved corresponds to the loss of Fitness score for were taken from Cancer Dependency Map (23). Supplementary Figure S2: Cellular targets of approved oncology drugs as excellent fitness genes in cancer-types for which drugs targeting these are not approved. Distribution of 43 cellular fitness genes with a significant loss of cellular fitness upon their depletion across peripheral nervous system, large intestine and ovarian cell lines