Proceedings of the XI International Symposium on Biological Control of Weeds Others (E.G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

27April12acquatic Plants
International Plant Protection Convention Protecting the world’s plant resources from pests 01 2012 ENG Aquatic plants their uses and risks Implementation Review and Support System Support and Review Implementation A review of the global status of aquatic plants Aquatic plants their uses and risks A review of the global status of aquatic plants Ryan M. Wersal, Ph.D. & John D. Madsen, Ph.D. i The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of speciic companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned.All rights reserved. FAO encourages reproduction and dissemination of material in this information product. Non-commercial uses will be authorized free of charge, upon request. Reproduction for resale or other commercial purposes, including educational purposes, may incur fees. Applications for permission to reproduce or disseminate FAO copyright materials, and all queries concerning rights and licences, should be addressed by e-mail to [email protected] or to the Chief, Publishing Policy and Support Branch, Ofice of Knowledge Exchange, -

The Role of Biological Control Agents in an IWM Program for Chrysanthemoides Monilifera Subsp
The role of biological control agents in an IWM program for Chrysanthemoides monilifera subsp. rotundata (bitou bush) Royce H. Holtkamp1 Summary Bitou bush, Chrysanthemoides monilifera subspecies rotundata, is a native of South Africa, which was used extensively in Australia as a sand-stabilising plant and for revegetation of coastal areas mined for mineral sands. It has now become a serious environmental weed in eastern Australia, primarily of conservation areas, where it significantly reduces biodiversity. Since 1989, six species of insects have been released on bitou bush, four of which have established. These are having varied impacts on bitou bush with bitou tip moth, Comostolopsis germana, and bitou seed fly, Mesoclanis polana, being the most successful. An integrated weed management approach appears to be the best option for long-term sustainable control of bitou bush. This paper discusses the use of biological control agents in combina- tion with other control options such as strategic herbicide applications, fire, physical removal and revegetation techniques. Keywords: biological control, bitou bush, Chrysanthemoides monilifera subspecies rotundata, integrated weed management. The plant withdrawn. However, this action came far too late and by 1976 C. monilifera subsp. rotundata was naturalised Chrysanthemoides monilifera subspecies rotundata along much of the NSW coast. (DC.) T. Norl. (bitou bush), is a competitive environ- A survey conducted in 2001 by the NSW National mental weed of South African origin. It is primarily Parks and Wildlife Service (NPWS) has shown C. restricted to areas of summer rainfall (Parsons and monilifera subsp. rotundata to be present on 900 km Cuthbertson 1992) and infests coastal areas of southern (80%) of the NSW coastline and the dominant plant on Queensland, New South Wales (NSW) and Lord Howe over 400 km. -

Field Host Range, Foraging Depth, and Impact Of
FIELD HOST RANGE, FORAGING DEPTH, AND IMPACT OF CRICOTOPUS LEBETIS SUBLETTE (DIPTERA: CHIRONOMIDAE), A BIOLOGICAL CONTROL AGENT OF HYDRILLA VERTICILLATA (L.F.) ROYLE (HYDROCHARITACEAE) By EUTYCHUS MUKURE KARIUKI A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2017 © 2017 Eutychus Mukure Kariuki To my loving family ACKNOWLEDGMENTS I would like to thank my Major Advisor, Dr. Raymond L. Hix, and my Co-Advisor, Dr. James P. Cuda, for their support and guidance during my Ph.D. program. I am also thankful to my committee members, Dr. Stephen D. Hight for his invaluable support and mentorship during the course of my research; Dr. Jennifer Gillett-Kaufman for her constant support, especially through the writing process of my dissertation; and Dr. Lyn Gettys for always being available to help with questions. I am grateful to all others who provided their assistance, including Dr. Edzard van Santen (University of Florida), Dr. Lazarus Mramba (University of Florida), Dr. Emma Weeks (University of Florida), John Mass (United States Department of Agriculture (USDA), Tallahassee, Florida), Kelle Sullivan (Florida Fish and Wildlife Conservation Commission), Dr. Lamberth Kanga (Florida A&M University), and Dr. Muhammad Haseeb (Florida A&M University). I am thankful to all my colleagues and lab mates at the University of Florida who reviewed this manuscript and offered valuable comments and suggestions. I am equally thankful to the USDA for providing funding to this study through the Hydrilla Integrated Pest Management Risk Avoidance and Mitigation Project (IPM RAMP) grant 2010-02825 and the National Institute of Food and Agriculture Crop Protection and Pest Management (NIFA CPPM) grant 2014-70006-22517. -

Appendix B Natural History and Control of Nonnative Invasive Species
Appendix B: Natural History and Control of Nonnative Invasive Plants Found in Ten Northern Rocky Mountains National Parks Introduction The Invasive Plant Management Plan was written for the following ten parks (in this document, parks are referred to by the four letter acronyms in bold): the Bear Paw Battlefield-BEPA (MT, also known as Nez Perce National Historical Park); Big Hole National Battlefield-BIHO (MT); City of Rocks National Reserve-CIRO (ID); Craters of the Moon National Monument and Preserve-CRMO (ID); Fossil Butte National Monument-FOBU (WY); Golden Spike National Historic Site-GOSP (UT); Grant-Kohrs Ranch National Historic Site-GRKO (MT); Hagerman Fossil Beds National Monument-HAFO (ID); Little Bighorn Battlefield National Monument-LIBI (MT); and Minidoka National Historic Site-MIIN (ID). The following information is contained for each weed species covered in this document (1) Park presence: based on formal surveys or park representatives’ observations (2) Status: whether the plant is listed as noxious in ID, MT, UT, or WY (3) Identifying characteristics: key characteristics to aid identification, and where possible, unique features to help distinguish the weed from look-a-like species (4) Life cycle: annual, winter-annual, biennial, or perennial and season of flowering and fruit set (5) Spread: the most common method of spread and potential for long distance dispersal (6) Seeds per plant and seed longevity (when available) (7) Habitat (8) Control Options: recommendations on the effectiveness of a. Mechanical Control b. Cultural -

Biological Control of Miconia Calvescens with a Suite of Insect Herbivores from Costa Rica and Brazil
Biological control of Miconia calvescens with a suite of insect herbivores from Costa Rica and Brazil F.R. Badenes-Perez,1,2 M.A. Alfaro-Alpizar,3 A. Castillo-Castillo3 and M.T. Johnson4 Summary Miconia calvescens DC. (Melastomataceae) is an invasive tree considered the most serious threat to the natural ecosystems of Hawaii and other Pacific islands. We evaluated nine species of natural enemies that feed on inflorescences or leaves ofM. calvescens for their potential as biological control agents, comparing their impact on the target plant, host specificity, and vulnerability to biotic inter- ference. Among herbivores attacking reproductive structures of M. calvescens, a fruit-galling wasp from Brazil, Allorhogas sp. (Hymenoptera: Braconidae), and a flower- and fruit-feeding moth from Costa Rica, Mompha sp. (Lepidoptera: Momphidae), were the most promising agents studied. The sawflyAtomacera petroa Smith (Hymenoptera: Argidae) from Brazil was thought to have the highest potential among the defoliators evaluated. Keywords: herbivory, host specificity, biotic interference. Introduction ductive structures may be necessary to achieve effec- tive biological control. Miconia calvescens DC. (Melastomataceae) is a small Several insects and pathogens have been identified tree native to Central and South America that is consid- as potential agents in surveys conducted in the native ered a serious threat to natural ecosystems in Hawaii range of M. calvescens in Brazil and Costa Rica by and other Pacific islands because of its ability to invade Johnson (unpublished data) and others (Burkhart, 1995; native forests (Medeiros et al., 1997). Its devastating Barreto et al., 2005; Picanço et al., 2005). In the inter- effects are most evident in Tahiti, where it has displaced est of avoiding unnecessary introductions and making over 65% of the native forest and threatens many en- efficient use of limited resources to evaluate potential demic species (Meyer and Florence, 1996). -

First Record of the Sedge Feeder Bactra Verutana Zeller (Lepidoptera
Revista Brasileira de Entomologia 63 (2019) 104–107 REVISTA BRASILEIRA DE Entomologia A Journal on Insect Diversity and Evolution www.rbentomologia.com Short Communication First record of the sedge feeder Bactra verutana Zeller (Lepidoptera: Tortricidae) in Chile based on morphology and DNA barcodes a,∗ b Héctor A. Vargas , Marcelo Vargas-Ortiz a Universidad de Tarapacá, Facultad de Ciencias Agronómicas, Departamento de Recursos Ambientales, Arica, Chile b Universidad de Concepción, Facultad de Ciencias Naturales y Oceanográficas, Departamento de Zoología, Programa de Doctorado en Sistemática y Biodiversidad Concepción, Chile a r a b s t r a c t t i c l e i n f o Article history: The sedge-feeding moth Bactra verutana Zeller, 1875 (Lepidoptera: Tortricidae: Olethreutinae: Bactrini), Received 4 October 2018 described from Dallas, Texas, USA, is widespread, recorded throughout much North America, Central Accepted 27 February 2019 and South America, including the Caribbean, and Africa. The species is recorded for the first time from Available online 21 March 2019 Chile based on specimens collected in the coastal valleys of the Atacama Desert, where its larvae feed Associate Editor: Livia Pinheiro on Cyperus corymbosus Rottb. var. subnodosus (Nees & Meyen) Kük. (Cyperaceae). A single DNA barcode haplotype, which is widespread in USA, was found in two Chilean specimens sequenced. Keywords: © 2019 Sociedade Brasileira de Entomologia. Published by Elsevier Editora Ltda. This is an open Atacama Desert Cyperaceae access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Cyperus corymbosus DNA barcoding Bactra Stephens, 1834 (Olethreutinae: Bactrini) is a widespread sequences (sensu Hebert et al., 2003) were used to assess the rela- genus of Tortricidae (Lepidoptera) with 106 described species tionships of the Chilean specimens. -

Modelling Bitou Bush (Chrysanthemoides Monilifera Ssp. Rotundata) and a Seed fly (Mesoclanis Polana)
Exploring interactions between cultural and biological control techniques: modelling bitou bush (Chrysanthemoides monilifera ssp. rotundata) and a seed fly (Mesoclanis polana) Darren J. Kriticos,1,3 Rachel M. Stuart1,2 and Julian E. Ash2 Summary Weed seed-production and seedbank dynamics have been a focus of attention for many biological control campaigns. This interest has perhaps been promoted by the recognition of the important role of weed seed dynamics in annual cropping systems, and frequent observations that seed production is markedly increased in ranges into which a plant is introduced, compared with rates in its native range. Seeds are the means by which most higher-order perennial plants disperse, and reestablish following disturbance. The role and importance of seeds in the population dynamics of weed popula- tions depends upon factors such as successional state of the invaded vegetation association, the distur- bance frequency, plant age at maturity, seed decay rate, and self-thinning patterns. The role of seeds and their predators in maintaining a plant population may be minimal, and decreasing the rate of seed production and the size of the seedbank may have only minor impacts on the population dynamics of perennial weeds. The interactions between cultural management techniques for bitou bush and its seed fly were explored using a process-based population dynamics model. The role of the seed fly in reducing the invasive potential of bitou bush and modifying the population reestablishment rates following distur- bance were studied. The seed fly has substantially reduced seed production, but the effect of the fly on canopy cover of bitou bush and on its invasion potential appears negligible. -
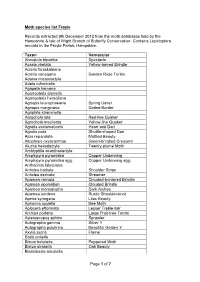
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Bioseries 06 2007.Pdf
Invasive alien flora and fauna in South Africa: expertise and bibliography by Charles F. Musil & Ian A.W. Macdonald Pretoria 2007 SANBI Biodiversity Series The South African National Biodiversity Institute (SANBI) was established on 1 September 2004 through the signing into force of the National Environmental Management: Biodiversity Act (NEMBA) No. 10 of 2004 by President Thabo Mbeki. The Act expands the mandate of the former National Botanical Institute to include responsibilities relating to the full diversity of South Africa’s fauna and flora, and builds on the internationally respected programmes in conservation, research, education and visitor services developed by the National Botanical Institute and its predecessors over the past century. The vision of SANBI is to be the leading institution in biodiversity science in Africa, facilitating conservation, sustainable use of living resources, and human wellbeing. SANBI’s mission is to promote the sustainable use, conservation, appreciation and enjoyment of the exceptionally rich biodiversity of South Africa, for the benefit of all people. SANBI Biodiversity Series publishes occasional reports on projects, technologies, workshops, symposia and other activities initiated by or executed in partnership with SANBI. Technical editor: Gerrit Germishuizen and Emsie du Plessis Design & layout: Daleen Maree Cover design: Sandra Turck The authors: C.F. Musil—Senior Specialist Scientist, Global Change & Biodiversity Program, South African National Biodiversity Institute, Private Bag X7, Claremont, 7735 ([email protected]) I.A.W. Macdonald—Extraordinary Professor, Sustainability Institute, School of Public Management and Planning, Stellenbosch University ([email protected]) How to cite this publication MUSIL, C.F. & MACDONALD, I.A.W. 2007. Invasive alien flora and fauna in South Africa: expertise and bibliography. -

Biological Control
Salsola tragus Biological Control: Hasan et al . (2001) report that, "The rust fungus Uromyces salsolae Reichardt (Isolate MW338; IMI No. 372660) was found on S. tragus in western Turkey. The attacked plants were covered with a powdery brown mass of unicellular, globular to oval-shaped urediniospores produced in round to elongated sori on the leaves and stems, and showed much reduced growth. Later in the season, the plants produced unicellular, thick-walled, dark teliospores borne in round to elongated telia. Similarly, the S. tragus plants from the USA, when inoculated in the greenhouse with a water suspension of the urediniospores of U. salsolae , developed brown uredinia and then telia as the disease advanced. The rust has been reported on several species of Salsola in the former-USSR, Israel, Iran, Romania, Australia, France, Pakistan, and Portugal (CAB International Report, unpublished). IMI records also show that the rust has been recorded on other genera of Chenopodiaceae from former USSR, Cyprus, and Romania. During our host specicity studies, the strain of U. salsolae collected by S. Hasan in Turkey was restricted to S. tragus and did not infect any of the other 16 plant species or varieties belonging to six different families that were tested. The fungus severely infected S. tragus plants not only from the USA but also those from Montpellier (France) and Turkey, showing that the rust may not be restricted only to certain biotypes of the weed. The rust fungus, which is highly damaging and effective in killing or severely reducing the growth of the weed under greenhouse conditions, has recently been imported into the USA for further host specificity testing under quarantine conditions. -

Biology of the Bruchidae +6178
Ann. Rev. Entomol 1979. 24:449-73 Copyright @ 1979 by Annual Reviews Inc. All rights reserved BIOLOGY OF THE BRUCHIDAE +6178 B. J. Southgate Biology Department, Pest Infestation Control Laboratory, Ministry of Agriculture, Fisheries, and Food, Slough SL3 7HJ, Berks, England INTRODUCTION Species of Bruchidae breed in every continent except Antarctica. The larg est number of species live in the tropical regions of Asia, Africa, and Central and South America. Many species have obvious economic importance because they breed on grain legumes and consume valuable proteins that would otherwise be eaten by man. Other species, however, destroy seeds of an immense number of leguminous trees and shrubs, which, though they have no obvious economic value, stem the advance of the deserts into the marginal cultivated areas of the world. When this ecosystem is mismanaged by practices such as over grazing, then any organism that restricts the normal regeneration of seed lings will, in the long run, affect agriculture adversely. This has been demonstrated recently in some African and Middle Eastern semiarid zones (65). The present interest in the management of arid areas and in the introduc Annu. Rev. Entomol. 1979.24:449-473. Downloaded from www.annualreviews.org Access provided by Copyright Clearance Center on 11/01/20. For personal use only. tion of alternative tree species to provide timber, fodder, or shade has stimulated a detailed study of the ecology of some leguminous trees and shrubs that has revealed some deleterious effects of bruchid beetles on the seeds of these plants (42, 43, 59). It has also emphasized the inadequacy of our knowledge of the taxonomy and biology of these beetles. -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe.