Water's Hydrogen Bond Strength That Is Used in This Paper
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Heavy Water (D2O) on Human Pancreatic Tumor Cells
ANTICANCER RESEARCH 25: 3407-3412 (2005) Effects of Heavy Water (D2O) on Human Pancreatic Tumor Cells JOHANNES HARTMANN1, YVONNE BADER1, ZSUZSANNA HORVATH1, PHILIPP SAIKO1, MICHAEL GRUSCH1, CHRISTOPH ILLMER1, SIBYLLE MADLENER1, MONIKA FRITZER-SZEKERES1, NICOLE HELLER2, RUDOLF-GIESBERT ALKEN2 and THOMAS SZEKERES1 1Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Vienna, General Hospital of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; 2BDD Berolina Drug Development GmbH, Fontanestrasse 84-89, D-15366 Neuenhagen, Germany Abstract. Background: Pancreatic cancer constitutes an carcinomas be removed by surgery. Individuals suffering entity which is difficult to treat and, therefore, mostly fatal. from inoperable tumors receive palliative therapy, including Since heavy water (deuterium oxide, D2O) was shown to be chemotherapy and radiation therapy. However, the active in various cancer cell lines in vitro and in vivo, we now treatment options are very limited and most patients die investigated its effects in human pancreatic tumor cells. within months after diagnosis. Materials and Methods: The cytotoxic effects of D2O were Incubation of tumor cells with various concentrations of examined in three pancreatic cancer cell lines (AsPC-1, D2O leads to inhibition of cell proliferation and might, BxPC-3 and PANC-1). Induction of apoptosis was therefore, help in the chemotherapeutic treatment of human determined by Hoechst/propidium iodide double staining and tumors (1). D2O, known as heavy water, contains a neutron cell cycle distribution was investigated by FACS analysis. and a proton in its hydrogen atoms and shows a variety of Results: Employing a clonogenic assay, D2O yielded IC50 different biological activities from normal (light) water. -

An Alternate Graphical Representation of Periodic Table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt
An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. [email protected] Abstract Periodic table of chemical elements symbolizes an elegant graphical representation of symmetry at atomic level and provides an overview on arrangement of electrons. It started merely as tabular representation of chemical elements, later got strengthened with quantum mechanical description of atomic structure and recent studies have revealed that periodic table can be formulated using SO(4,2) SU(2) group. IUPAC, the governing body in Chemistry, doesn‟t approve any periodic table as a standard periodic table. The only specific recommendation provided by IUPAC is that the periodic table should follow the 1 to 18 group numbering. In this technical paper, we describe a new graphical representation of periodic table, referred as „Circular form of Periodic table‟. The advantages of circular form of periodic table over other representations are discussed along with a brief discussion on history of periodic tables. 1. Introduction The profoundness of inherent symmetry in nature can be seen at different depths of atomic scales. Periodic table symbolizes one such elegant symmetry existing within the atomic structure of chemical elements. This so called „symmetry‟ within the atomic structures has been widely studied from different prospects and over the last hundreds years more than 700 different graphical representations of Periodic tables have emerged [1]. Each graphical representation of chemical elements attempted to portray certain symmetries in form of columns, rows, spirals, dimensions etc. Out of all the graphical representations, the rectangular form of periodic table (also referred as Long form of periodic table or Modern periodic table) has gained wide acceptance. -

Power Sources Challenge
POWER SOURCES CHALLENGE FUSION PHYSICS! A CLEAN ENERGY Summary: What if we could harness the power of the Sun for energy here on Fusion reactions occur when two nuclei come together to form one Earth? What would it take to accomplish this feat? Is it possible? atom. The reaction that happens in the sun fuses two Hydrogen atoms together to produce Helium. It looks like this in a very simplified way: Many researchers including our Department of Energy scientists and H + H He + ENERGY. This energy can be calculated by the famous engineers are taking on this challenge! In fact, there is one DOE Laboratory Einstein equation, E = mc2. devoted to fusion physics and is committed to being at the forefront of the science of magnetic fusion energy. Each of the colliding hydrogen atoms is a different isotope of In order to understand a little more about fusion energy, you will learn about hydrogen, one deuterium and one the atom and how reactions at the atomic level produce energy. tritium. The difference in these isotopes is simply one neutron. Background: It all starts with plasma! If you need to learn more about plasma Deuterium has one proton and one physics, visit the Power Sources Challenge plasma activities. neutron, tritium has one proton and two neutrons. Look at the The Fusion Reaction that happens in the SUN looks like this: illustration—do you see how the mass of the products is less than the mass of the reactants? That is called a mass deficit and that difference in mass is converted into energy. -

Deuterium As a Research Tool in the Physical and Biological Sciences
DEUTERIUM AS A RESEARCH TOOL IN THE PHYSICAL AND BIOLOGICAL SCIENCES HERRICK L. JOHNSTON Ohio State University The discovery (1) of deuterium and the production of "heavy water," its chief compound, in a nearly pure state (2), are among the more important scientific achievements of recent years. In less than two years from the production of heavy water in nearly pure condition over three hundred papers reporting investigations on or with deuterium have appeared in scientific journals. While most of these investiga- tions have been of a physical or chemical nature significant results have been reported in investigations of biological character. It is probable that the principal role of deuterium, in future research in all of these fields, will be more that of a research tool than as an object of investigation. Deuterium is not a new chemical element, as the name might imply, but is a special variety of hydrogen atom. It differs from the ordinary (or light) hydrogen, chiefly, in mass. The atomic weight of the ordinary hydrogen is one while that of deuterium, or heavy hydrogen, is two. It resembles the ordinary hydrogen atom in possessing just one unit of positive charge on its nucleus (equal to the number of electrons in the neutral atom) and it is this latter property which determines the chemical character of an atom, and hence its position in the family of elements. The existence of atoms which differ in mass although alike in nuclear charge is common among the elements, and atomic species which are related in this manner are called isotopes. -

HEAVY WATER and NONPROLIFERATION Topical Report
HEAVY WATER AND NONPROLIFERATION Topical Report by MARVIN M. MILLER MIT Energy Laboratory Report No. MIT-EL 80-009 May 1980 COO-4571-6 MIT-EL 80-009 HEAVY WATER AND NONPROLIFERATION Topical Report Marvin M. Miller Energy Laboratory and Department of Nuclear Engineering Massachusetts Institute of Technology Cambridge, Massachusetts 02139 May 1980 Prepared For THE U.S. DEPARTMENT OF ENERGY UNDER CONTRACT NO. EN-77-S-02-4571.A000 NOTICE This report was prepared as an account of work sponsored by the United States Government. Neither the United States nor the United States Department of Energy, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or useful- ness of any information, apparatus, product or process disclosed or represents that its use would not infringe privately owned rights. A B S T R A C T The following report is a study of various aspects of the relationship between heavy water and the development of the civilian and military uses of atomic energy. It begins with a historical sketch which traces the heavy water storyfrom its discovery by Harold Urey in 1932 through its coming of age from scientific curiosity to strategic nuclear material at the eve of World War II and finally into the post-war period, where the military and civilian strands have some- times seemed inextricably entangled. The report next assesses the nonproliferation implications of the use of heavy water- moderated power reactors; several different reactor types are discussed, but the focus in on the natural uranium, on- power fueled, pressure tube reactor developed in Canada, the CANDU. -

Application of Aluminium Oxide Nanoparticles to Enhance Rheological and Filtration Properties of Water Based Muds at HPHT Condit
Application of Aluminium Oxide Nanoparticles to Enhance Rheological and Filtration Properties of Water Based Muds at HPHT Conditions Sean Robert Smith1, Roozbeh Rafati*1, Amin Sharifi Haddad1, Ashleigh Cooper2, Hossein Hamidi1 1School of Engineering, University of Aberdeen, Aberdeen, United Kingdom, AB24 3UE 2Baroid Drilling Fluid Laboratory, Halliburton, Dyce, Aberdeen, United Kingdom, AB21 0GN Abstract Drilling fluid is of one of the most important elements of any drilling operation, and through ever advancing technologies for deep water and extended reach drillings, enhancement of drilling fluids properties for such harsh conditions need to be investigated. Currently oil based fluids are used in these types of advanced drilling operations, as their performance at high pressure and high temperature conditions, and deviated wells are superior compared to water based fluids. However, the high costs associated with using them, and environmental concerns of such oil base fluids are drawbacks of them at the moment. In this study, we tried to find a solution for such issues through investigation on the potential application of a new nano-enhanced water base fluid for advanced drilling operations. We analysed the performance of water base drilling fluids that were formulated with two type of nanomaterials (aluminium oxide and silica) at both high and low pressure and temperature conditions (high: up to 120 oC and 500 psi, low: 23 oC and 14.7 psi). The results were compared with a base case drilling fluid with no nanomaterial, and they showed that there is an optimum concentration for aluminium oxide nanoparticles that can be used to improve the rheological and filtration properties of drilling fluids. -

Tritium Immobilization and Packaging Using Metal Hydrides
AECL-71S1 ATOMIC ENERGY flPnSy L'ENERGIE ATOMIQUE OF CANADA LIMITED V^^JP DU CANADA LIMITEE TRITIUM IMMOBILIZATION AND PACKAGING USING METAL HYDRIDES Immobilisation et emballage du tritium au moyen d'hydrures de meta! W.J. HOLTSLANDER and J.M. YARASKAVITCH Chalk River Nuclear Laboratories Laboratoires nucl6aires de Chalk River Chalk River, Ontario April 1981 avril ATOMIC ENERGY OF CANADA LIMITED Tritium Immobilization and Packaging Using Metal Hydrides by W.J. Holtslander and J.M. Yaraskavitch Chemical Engineering Branch Chalk River Nuclear Laboratories Chalk River, Ontario KOJ 1J0 1981 April AECL-7151 L'ENERGIE ATOMIQUE DU CANADA, LIMITEE Immo ;ilisation et emballage du tritium au moyen d'hydrures de mëtâT par W.J. Holtslander et J.M. Yaraskavitch Résumé Le tritium provenant des réacteurs CANDU â eau lourde devra être emballé et stocké de façon sûre. Il sera récupéré sous la forme élémentaire T2. Les tritiures de métal sont des composants efficaces pour immobiliser le tritium comme solide non réactif stable et ils peuvent en contenir beaucoup. La technologie nécessaire pour préparer les hydrures des métaux appropriés, comme le titane et le zirconium,a été développée et les propriétés des matériaux préparés ont été évaluées. La conception des emballages devant contenir les tritiures de métal, lors du transport et durant le stockage à long terme, est terminée et les premiers essais ont commencé. Département de génie chimique Laboratoires nucléaires de Chalk River Chalk River, Ontario KOJ 1J0 Avril 1981 AECL-7151 ATOMIC ENERGY OF CANADA LIMITED Tritium Immobilization and Packaging Using Metal Hydrides by W.J. Holtslander and J.M. -

2.#Water;#Acid.Base#Reac1ons
8/24/15 BIOCH 755: Biochemistry I Fall 2015 2.#Water;#Acid.base#reac1ons# Jianhan#Chen# Office#Hour:#M#1:30.2:30PM,#Chalmers#034# Email:#[email protected]# Office:#785.2518# 2.1#Physical#Proper1es#of#Water# • Key$Concepts$2.1$ – Water#molecules,#which#are#polar,#can#form#hydrogen#bonds#with# other#molecules.# – In#ice,#water#molecules#are#hydrogen#bonded#in#a#crystalline#array,#but# in#liquid#water,#hydrogen#bonds#rapidly#break#and#re.form#in#irregular# networks.# – The#aTrac1ve#forces#ac1ng#on#biological#molecules#include#ionic# interac1ons,#hydrogen#bonds,#and#van#der#Waals#interac1ons.# – Polar#and#ionic#substances#can#dissolve#in#water.# (c)#Jianhan#Chen# 2# 1 8/24/15 2.1#Physical#Proper1es#of#Water# • Key$Concepts$2.1$ – The#hydrophobic#effect#explains#the#exclusion#of#nonpolar#groups#as#a# way#to#maximize#the#entropy#of#water#molecules.# – Amphiphilic#substances#form#micelles#or#bilayers#that#hide#their# hydrophobic#groups#while#exposing#their#hydrophilic#groups#to#water.# – Molecules#diffuse#across#membranes#which#are#permeable#to#them# from#regions#of#higher#concentra1on#to#regions#of#lower# concentra1on.# – In#dialysis,#solutes#diffuse#across#a#semipermeable#membrane#from# regions#of#higher#concentra1on#to#regions#of#lower#concentra1on.# (c)#Jianhan#Chen# 3# Human#Body#Mass#Composi1on# (c)#Jianhan#Chen# 4 2 8/24/15 Structure#of#Water# (c)#Jianhan#Chen# 5 Water#Hydrogen#Bonding# ~1.8 Å, 180o Acceptor Donor (c)#Jianhan#Chen# 6# 3 8/24/15 Typical#Bond#Energies# (c)#Jianhan#Chen# 7# Hydrogen#bond#networks#of#water/ice# (c)#Jianhan#Chen# -

Physical-Chemical Properties of Complex Natural Fluids
Physical-chemical properties of complex natural fluids Vorgelegt von Diplom-Geochemiker Diplom-Mathematiker Sergey Churakov aus Moskau an der Fakultät VI - Bauingenieurwesen und Angewandte Geowissenschaften - der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktor der Naturwissenschaften - Dr. rer. nat. - genehmigte Dissertation Berichter: Prof. Dr. G. Franz Berichter: Prof. Dr. T. M. Seward Berichter: PD. Dr. M. Gottschalk Tag der wissenschaftlichen Aussprache: 27. August 2001 Berlin 2001 D83 Abstract The dissertation is focused on the processes of transport and precipitation of metals in high temperature fumarole gases (a); thermodynamic properties of metamorphic fluids at high pressures (b); and the extent of hydrogen-bonding in supercritical water over wide range of densities and temperatures (c). (a) At about 10 Mpa, degassing of magmas is accompanied by formation of neary ‘dry’ salt melts as a second fluid phase, very strong fractionation of hydrolysis products between vapour and melts, as well as subvalence state of metals during transport processes. Based on chemical analyses of gases and condensates from high-temperature fumaroles of the Kudryavy volcano (i Iturup, Kuril Arc, Russia), a thermodynamic simulation of transport and deposition of ore- and rock-forming elements in high-temperature volcanic gases within the temperature range of 373-1373 K at 1 bar pressure have been performed. The results of the numerical simulations are consistent with field observations. Alkali and alkali earth metals, Ga, In, Tl, Fe, Co, Ni, Cu, and Zn are mainly transported as chlorides in the gas phase. Sulfide and chloride forms are characteristic of Ge, Sn, Pb, and Bi at intermediate and low temperatures. -

Impact of Solute Molecular Properties on the Organization of Nearby Water: a Cellular Automata Model
Digest Journal of Nanomaterials and Biostructures Vol. 7, No. 2, April - June 2012, p. 469 - 475 IMPACT OF SOLUTE MOLECULAR PROPERTIES ON THE ORGANIZATION OF NEARBY WATER: A CELLULAR AUTOMATA MODEL C. MIHAIa, A. VELEAb,e, N. ROMANc, L. TUGULEAa, N. I. MOLDOVANd** aDepartment of Electricity, Solid State and Biophysics, Faculty of Physics, University of Bucharest, Romania bNational Institute of Materials Physics, Bucharest - Magurele, Romania cDepartment of Computer Science, The Ohio State University - Lima, Ohio, USA dDepartment of Internal Medicine, Davis Heart and Lung Research Institute, The Ohio State University - Columbus, Ohio, USA e"Horia Hulubei” Foundation, Bucharest-Magurele, Romania The goal of this study was the creation of a model to understand how solute properties influence the structure of nearby water. To this end, we used a two-dimensional cellular automaton model of aqueous solutions. The probabilities of translocation of water and solute molecules to occupy nearby sites, and their momentary distributions (including that of vacancies), are considered indicative of solute molecular mechanics and hydrophatic character, and are reflected in water molecules packing, i.e. ‘organization’. We found that in the presence of hydrophilic solutes the fraction of water molecules with fewer neighbors was dominant, and inverse-proportionally dependent on their relative concentration. Hydrophobic molecules induced water organization, but this effect was countered by their own flexibility. These results show the emergence of cooperative effects in the manner the molecular milieu affects local organization of water, and suggests a mechanism through which molecular mechanics and crowding add a defining contribution to the way the solute impacts on nearby water. -

Unique Properties of Water!
Name: _______ANSWER KEY_______________ Class: _____ Date: _______________ Unique Properties of Water! Word Bank: Adhesion Evaporation Polar Surface tension Cohesion Freezing Positive Universal solvent Condensation Melting Sublimation Dissolve Negative 1. The electrons are not shared equally between the hydrogen and oxygen atoms of water creating a Polar molecule. 2. The polarity of water allows it to dissolve most substances. Because of this it is referred to as the universal solvent 3. Water molecules stick to other water molecules. This property is called cohesion. 4. Hydrogen bonds form between adjacent water molecules because the positive charged hydrogen end of one water molecule attracts the negative charged oxygen end of another water molecule. 5. Water molecules stick to other materials due to its polar nature. This property is called adhesion. 6. Hydrogen bonds hold water molecules closely together which causes water to have high surface tension. This is why water tends to clump together to form drops rather than spread out into a thin film. 7. Condensation is when water changes from a gas to a liquid. 8. Sublimation is when water changes from a solid directly to a gas. 9. Freezing is when water changes from a liquid to a solid. 10. Melting is when water changes from a solid to a liquid. 11. Evaporation is when water changes from a liquid to a gas. 12. Why does ice float? Water expands as it freezes, so it is LESS DENSE AS A SOLID. 13. What property refers to water molecules resembling magnets? How are these alike? Polar bonds create positive and negative ends of the molecule. -
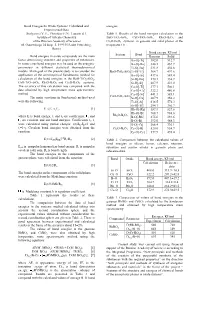
Bond Energies in Oxide Systems: Calculated and Experimental Data
Bond Energies In Oxide Systems: Calculated and energies. Experimental Data Stolyarova V. L., Plotnikov E.N., Lopatin S.I. Table 1. Results of the bond energies calculation in the Institute of Silicate Chemistry BaO-TiO 2-SiO 2, CaO-TiO 2-SiO 2, Rb 2O-B2O3 and of the Russian Academy of Sciences Cs 2O-B2O3 systems. in gaseous and solid phases at the ul. Odoevskogo 24 korp. 2, 199155 Sankt Petersburg, temperature 0 ¥ . Russia Bond energy, KJ/mol System Bond Bond energies in oxide compounds are the main Gaseous Solid factor determining structure and properties of substances. Ba-O[-Ti] 382.0 512.7 In some cases bond energies may be used as the energetic Ba-O[-Ba] 348.5 467.7 parameters in different statistical thermodynamical Ti-O[-Ba] 331.8 450.6 models. Main goal of the present study is to consider the BaO-TiO 2-SiO 2 Ti-O[-Ti] 364.7 497.6 application of the semiempirical Sanderson's method for Ba-O[-Si] 437.6 585.4 calculation of the bond energies in the BaO-TiO 2-SiO 2, Si-O[-Ba] 270.3 334.7 CaO-TiO 2-SiO 2, Rb 2O-B2O3 and Cs 2O-B2O3 systems. Si-O[-Si] 407.9 421.0 The accuracy of this calculation was compared with the Ca-O[-Ti] 377.1 500.1 data obtained by high temperature mass spectrometric Ca-O[-Ca] 322.2 486.8 method. Ca-O[-Si] 441.1 585.1 CaO-TiO -SiO The main equations in Sanderson's method used 2 2 Si-O[-Ca] 287.9 358.2 were the following.