Advanced Liver Disease SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Practice Parameters for the Treatment of Patients with Dominantly Inherited Colorectal Cancer
Practice Parameters For The Treatment Of Patients With Dominantly Inherited Colorectal Cancer Diseases of the Colon & Rectum 2003;46(8):1001-1012 Prepared by: The Standards Task Force The American Society of Colon and Rectal Surgeons James Church, MD; Clifford Simmang, MD; On Behalf of the Collaborative Group of the Americas on Inherited Colorectal Cancer and the Standards Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons is dedicated to assuring high quality patient care by advancing the science, prevention, and management of disorders and diseases of the colon, rectum, and anus. The standards committee is composed of Society members who are chosen because they have demonstrated expertise in the specialty of colon and rectal surgery. This Committee was created in order to lead international efforts in defining quality care for conditions related to the colon, rectum, and anus. This is accompanied by developing Clinical Practice Guidelines based on the best available evidence. These guidelines are inclusive, and not prescriptive. Their purpose is to provide information on which decisions can be made, rather than dictate a specific form of treatment. These guidelines are intended for the use of all practitioners, health care workers, and patients who desire information about the management of the conditions addressed by the topics covered in these guidelines. Practice Parameters for the Treatment of Patients With Dominantly Inherited Colorectal Cancer Inherited colorectal cancer includes two main syndromes in which predisposition to the disease is based on a germline mutation that may be transmitted from parent to child. -

Clinical Audit on Management of Hematemesis in Children Admitted to Pediatric Gastroenterology and Hepatology Unit of Assiut
Med. J. Cairo Univ., Vol. 86, No. 8, December: 4531-4536, 2018 www.medicaljournalofcairouniversity.net Clinical Audit on Management of Hematemesis in Children Admitted to Pediatric Gastroenterology and Hepatology Unit of Assiut University Children Hospital ESRAA T. AHMED, M.Sc.; FATMA A. ALI, M.D. and NAGLA H. ABU FADDAN, M.D. The Department of Pediatrics, Faculty of Medicine, Assiut University, Assiut, Egypt Abstract Hematemesis: Indicates that the bleeding origin is above the Treitz angle, i.e., that it constitutes an Background: Hematemesis is an uncommon but potentially Upper Gastrointestinal Bleeding (UGIB) [3] . serious and life-threatening clinical condition in children. It indicates that the bleeding origin is above the Treitz angle, The etiology of upper GI bleeding varies by i.e., that it constitutes an Upper Gastrointestinal Bleeding (UGIB). age. The pathophysiology of upper GI bleeding is related to the source of the bleeding. Most clinically Aim of Study: To assess for how much the adopted proto- significant causes of upper GI bleeds are associated cols of management of children with upper gastrointestinal bleeding were applied at Gastroenterology & Hepatology Unit with ulcers, erosive esophagitis, gastritis, varices, of Assiut University Children Hospital. and/or Mallory-Weiss tears. While Physiologic Patients and Methods: This study is a an audit on man- stress, NSAIDs such as aspirin and ibuprofen, and agement of children with upper gastrointestinal bleeding infection with Helicobacter pylori are few of the admitted to pediatric Gastroenterology and Hepatology Unit, factors contributing to the imbalance leading to Assiut University Children Hospital during the period from ulcers and erosions in the GI tract [4] . -

Endoscopy Rotation Coordination and Goals and Objects Department of Surgery Stanford School of Medicine (8/15/17, Jnl)
Endoscopy Rotation Coordination And Goals and Objects Department of Surgery Stanford School of Medicine (8/15/17, jnl) Rotation Director: James Lau, MD ATTENDINGS and CONTACT INFORMATION Cell Phone E-mail Address James Lau, MD (702) 306-8780 [email protected] Homero Rivas, MD MBA (972) 207-2381 [email protected] Dan Azagury, MD (650) 248-3173 [email protected] Shai Friedland, MD [email protected] Andrew Shelton, MD [email protected] Natalie Kirilcuk, MD [email protected] Cindy Kin, MD [email protected] Laren Becker, MD [email protected] Jennifer Pan, MD [email protected] Suzanne Matsui, MD [email protected] Ramsey Cheung, MD [email protected] KEYPOINT The key for this rotation is that you need to show initiative. TEXT Practical Gastrointestinal Endoscopy: The Fundamentals. Sixth Edition. By Peter B. Cotton, Christopher B. Williams, Robert H. Hawes and Brian P. Saunders. You are responsible for the material to enhance your understanding and supplement your past experiences. Lots of pictures and tips and tricks. Quick read. Copy of text available for purchase on Amazon.com or for check out from the Lane Library. Procedure Schedule Monday Tuesday Wednesday Thursday Friday Laren Becker Jennifer Pan Shelton/Kirilcuk/Kin Ramsey Suzanne (VA (VA Colonoscopy 8:00 am Cheung (VA Matsui (VA Livermore) Livermore) (Stanford Endoscopy) Livermore) Livermore) Every other Tuesday Rivas/Lau alternating Upper/Occasional Lower 1 Endoscopy 9a-1p (Stanford Endoscopy) Suzanne Matsui (VA Livermore) The Staff Drs. Becker, Cheung, Pan, and Matsui are gastroenterologists that perform 75% colonoscopies and 25% upper endoscopies at the Livermore location for the Palo Alto VA. -

Chronic Diarrhea
Chronic Diarrhea Barbara McElhanon, MD Subra Kugathasan, MD Emory University School of Medicine 2013 Resident Education Series Reviewed by Edward Hoffenberg, MD of the Professional Education Committee Case • A 15 year old boy with PMH of obesity, anxiety disorder & ADHD presents with 3 months of non-bloody loose stool 5-15 times/day and diffuse abdominal pain that is episodically severe Case - History • Wellbutrin was stopped prior to the onset of her symptoms and her Psychiatrist was weaning Cymbalta • After stopping Cymbalta, she went to Costa Rica for a month long medical mission trip • Started having symptoms of abdominal pain and diarrhea upon return from her trip. • Ingestion of local Georgia creek water, but after her symptoms had started • Subjective fever x 4 days Case - Lab work by PCP • At onset of illness: – + occult blood in stool – + stool calprotectin (a measure of inflammation in the colon) – Negative stool WBC – Negative stool culture – Negative C. difficile – Negative ova & parasite study – Negative giardia antigen – Normal CBC with diff, Complete metabolic panel, CRP, ESR Case - History • Non-bloody diarrhea and abdominal pain continues • No relation to food • No fevers • No weight loss • Normal appetite • No night time occurrences • No other findings on ROS • No sick contacts Case – Work-up prior to visit Labs Imaging and Procedures • MRI enterography (MRI of the • Fecal occult blood, stool abdomen/pelvis with special cuts calprotectin, stool WBC, stool to evaluate the small bowel) culture, stool O&P, stool giardia -

Hemosuccus Pancreaticus: a Rare Cause of Upper Gastrointestinal Bleeding During Pregnancy Rani Akhil Bhat,1 Vani Ramkumar,1 K
Hemosuccus Pancreaticus: A Rare Cause Of Upper Gastrointestinal Bleeding During Pregnancy Rani Akhil Bhat,1 Vani Ramkumar,1 K. Akhil Krishnanand Bhat, 2 Rajgopal Shenoy2 Abstract Upper gastrointestinal bleeding is most commonly caused by From the 1Department of Department of Obstetrics and Gynaecology, Oman Medical 2 lesions in the esophagus, stomach or duodenum. Bleeding which College, Sohar, Sultanate of Oman, Department of Surgery, Oman Medical College, Sohar, Sultanate of Oma. originates from the pancreatic duct is known as hemosuccus pancreaticus. Only a few scattered case reports of hemosuccus Received: 06 Nov 2009 pancreaticus during pregnancy have been recorded in literature. Accepted: 31 Dec 2009 This is a case of a primigravida with 37 weeks of gestation Address correspondence and reprint request to: Dr. Rani A. Bhat,Department of with hemosuccus pancreaticus and silent chronic pancreatitis. Obstetrics and Gynaecology, Oman Medical College, P. O. Box 391, P. C. 321, Al- Evaluating pregnant women with upper gastrointestinal Tareef, Sohar, Sultanate of Oman. bleeding differs from that of non pregnant women as diagnostic E-mail: [email protected] modalities using radiation cannot be used. Therefore, Esophagogastroduodenoscopy should be performed at the time of active bleeding to diagnose hemosuccus pancreaticus. Bhat RA, et al. OMJ. 25 (2010); doi:10.5001/omj.2010.21 Introduction examination showed a combination of dark red blood and melena. Laboratory investigations revealed hemoglobin of 6.3 grams/dL, Hemosuccus pancreaticus is the term used to describe the liver function tests, serum amylase, glucose and prothrombin time syndrome of gastrointestinal bleeding into the pancreatic duct were within the normal range. -

Colon and Rectal Surgery Case Log Instructions Review Committee for Colon and Rectal Surgery
Colon and Rectal Surgery Case Log Instructions Review Committee for Colon and Rectal Surgery Background The ACGME Case Log System is a data depository which provides a mechanism that supports programs in complying with requirements, and also provides a uniform mechanism to verify the clinical education of residents among programs. The Case Log System is designed to capture and categorize a resident’s experience with patient care. It was initially instituted in 2001, and the Review Committee for Colon and Rectal Surgery has required its use by accredited programs since 2005. It is the intention of the Review Committee for Colon and Rectal Surgery that each resident has a reasonably equivalent educational experience to prepare for the practice of the specialty. As part of the process, the case numbers for each resident completing a program are collected and analyzed. To accomplish this complex task, a structured database has been created using standard codes for diagnoses and procedures. The Case Log System helps assess the breadth and depth of clinical experience provided to each colon and rectal surgery resident by his or her program with the ultimate goal of improving the programs themselves. It is the responsibility of the individual residents to accurately and in a timely manner enter their case data. The data entered will be monitored by the program directors and analyzed by the Review Committee. Separate analysis reports are created annually for the Committee, for program directors, and for residents. Additionally, the ACGME provides information regarding individual residents’ experience to the American Board of Colon and Rectal Surgery (ABCRS) as one criterion for their admission to the ABCRS’s exam process. -

Gastrointestinal Illness (GI)
Gastrointestinal Illness (GI) Gastrointestinal illness (GI) is one of the most common causes of outbreaks in LTCFs. There are many causal agents for GI illnesses including: viruses like Hepatitis A and norovirus, bacteria like E. coli and Salmonella and parasites such as Giardia and Cryptosporidium. • Mode of Transmission: Person-to-person through the fecal-oral route, but can also be 2 transferred through contaminated food and objects • Symptoms: ◦ Bacteria – loss of appetite, nausea and vomiting, diarrhea, abdominal pain/cramps, blood in stool, fever ◦ Virus – watery diarrhea, nausea and vomiting, headache, muscle aches ◦ Ova and Parasites – diarrhea, mucous/blood in stool, nausea or vomiting, severe abdominal pain • Duration: Less than two weeks Gastrointestinal Illness: Any combination of diarrhea (≥ 3 loose stools in 24 hours), vomiting, abdominal pain, with or without fever Gastrointestinal Illness Outbreak: The occurrence of more cases of GI illness in a 24-hour period than would normally be expected based on a facility’s individual surveillance data Precautions • Practice proper hand hygiene. • Clean and disinfect contaminated surfaces. • Follow the CDC’s Standard Precautions guidelines. • Understand that any patient with foodborne illness may represent the sentinel case of a more widespread outbreak. • Communicate with patients about ways to prevent food-related diseases. • Wash fruits and vegetables and cook all food, including seafood thoroughly. • When you are sick, do not prepare food or care for others who are sick. • Wash laundry thoroughly. Reporting Process Upon suspicion of a GI outbreak, facilities are required to notify DOH-Collier at (239)252-8226. Once notified, DOH-Collier will provide initial guidance, educational materials and two forms (listed below). -
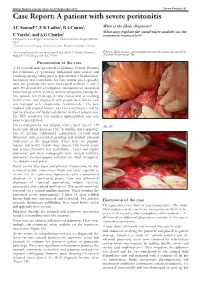
Case Report: a Patient with Severe Peritonitis
Malawi Medical Journal; 25(3): 86-87 September 2013 Severe Peritonitis 86 Case Report: A patient with severe peritonitis J C Samuel1*, E K Ludzu2, B A Cairns1, What is the likely diagnosis? 2 1 What may explain the small white nodules on the C Varela , and A G Charles transverse mesocolon? 1 Department of Surgery, University of North Carolina, Chapel Hill NC USA 2 Department of Surgery, Kamuzu Central Hospital, Lilongwe Malawi Corresponding author: [email protected] 4011 Burnett Womack Figure1. Intraoperative photograph showing the transverse mesolon Bldg CB 7228, Chapel Hill NC 27599 (1a) and the pancreas (1b). Presentation of the case A 42 year-old male presented to Kamuzu Central Hospital for evaluation of worsening abdominal pain, nausea and vomiting starting 3 days prior to presentation. On admission, his history was remarkable for four similar prior episodes over the previous five years that lasted between 3 and 5 days. He denied any constipation, obstipation or associated hematemesis, fevers, chills or urinary symptoms. During the first episode five years ago, he was evaluated at an outlying health centre and diagnosed with peptic ulcer disease and was managed with omeprazole intermittently . His past medical and surgical history was non contributory and he had no allergies and he denied alcohol intake or tobacco use. His HIV serostatus was negative approximately one year prior to presentation. On examination he was afebrile, with a heart rate of 120 (Fig 1B) beats/min, blood pressure 135/78 mmHg and respiratory rate of 22/min. Abdominal examination revealed mild distension with generalized guarding and marked rebound tenderness in the epigastrium. -

Esophageal Varices
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Crossref Hindawi Publishing Corporation Case Reports in Critical Care Volume 2016, Article ID 2370109, 4 pages http://dx.doi.org/10.1155/2016/2370109 Case Report A Rare but Reversible Cause of Hematemesis: (Downhill) Esophageal Varices Lam-Phuong Nguyen,1,2,3 Narin Sriratanaviriyakul,1,2,3 and Christian Sandrock1,2,3 1 Division of Pulmonary, Critical Care, and Sleep Medicine, University of California, Davis, Suite #3400, 4150 V Street, Sacramento, CA 95817, USA 2Department of Internal Medicine, University of California, Davis, Sacramento, USA 3VA Northern California Health Care System, Mather, USA Correspondence should be addressed to Lam-Phuong Nguyen; [email protected] Received 12 December 2015; Accepted 1 February 2016 Academic Editor: Kurt Lenz Copyright © 2016 Lam-Phuong Nguyen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. “Downhill” varices are a rare cause of acute upper gastrointestinal bleeding and are generally due to obstruction of the superior vena cava (SVC). Often these cases of “downhill” varices are missed diagnoses as portal hypertension but fail to improve with medical treatment to reduce portal pressure. We report a similar case where recurrent variceal bleeding was initially diagnosed as portal hypertension but later found to have SVC thrombosis presenting with recurrent hematemesis. A 39-year-old female with history of end-stage renal disease presented with recurrent hematemesis. Esophagogastroduodenoscopy (EGD) revealed multiple varices. -

Etiology of Upper Gastrointestinal Haemorrhage in a Teaching Hospital
TAJ June 2008; Volume 21 Number 1 ISSN 1019-8555 The Journal of Teachers Association RMC, Rajshahi Original Article Etiology of Upper Gastrointestinal Haemorrhage in a Teaching Hospital M Uddin Ahmed1, M Abdul Ahad2, M A Alim2, A R M Saifuddin Ekram3, Q Abdullah Al Masum4, Sumona Tanu5, Refaz Uddin6 Abstract A descriptive study on all cases of haematemesis and or melaena was carried out at Rajshahi Medical College Hospital to observe the demographic profile, clinical presentation, cause and outcome of upper gastrointestinal bleeding in a tertiary hospital of Bangladesh. Fifty adult patients presenting with haematemesis and or melaena admitted consecutively into medical unit were evaluated through proper history taking, thorough clinical examination, endoscopic examination with in 48 hours of first presentation and other related investigations. Patients those who were not stabilized haemodynamically with in 48 hours of resuscitation and endoscopy could not be done with in that period were excluded from this study. Results our results showed that out of 50 patients 44 were male and 6 were female and average age of the patients was 39.9 years. Most of the patients were from low socio-economic condition. Farmers, service holders and laborers were the most (57%) affected group. Haematemesis and melaena (42%), only melaena (42%) and only haematemesis (16%) were the presenting features. Endoscopy revealed that duodenal ulcer( 34%) was the most common cause of UGI bleeding followed by rupture of portal varices( 16%) , neoplasm( 10%) , gastric ulcer ( 08%) and gastric erosion( 06%). Acute upper GI bleeding is a common medical problem that is responsible for significant morbidity and mortality. -

Frequently Used Diagnosis Codes
Frequently Used Diagnosis Codes LIVER DIAGNOSIS CODES: Kidney/Pancreas Diagnosis Codes Abdominal pain, generalized R10.84 Abdominal pain, unspec site R10.84 Abdominal pain, RUQ R10.11 Diabetes type I (juvenile) controlled E10.9 Alpha-1-antitrypsin deficiency E88.01 Diabetes type II controlled E11.9 Ascites, other R18.8 Diabetic Nephrosis E11.21 Autoimmune disease, NOS M35.9 Kidney Transplant Complication T86.10 Biliary atresia Q44.2 Kidney Transplant, Failure T86.12 Blood in stool K92.1 Kidney Transplant, Infection T86.13 Cholangitis and/or PSC K83.0 Kidney Transplant, Rejection T86.11 Cirrhosis, Biliary (PBC-PSC) K74.5 Pancreas Transplant Complication T86.899 Cirrhosis, Liver w/o Alcohol K74.60 Renal Failure, ESRD N18.6 Cirrhosis, Liver w/Alcohol K70.30 Transplant-Kidney Status Post Z94.0 Colitis, Ulcerative Unspec K51.90 Transplant -Pancreas Status Post Z94.83 Crohn's Disease, large intestine K50.10 Cystic disease, liver, congential Q44.6 Heart/Lung Diagnosis Codes Encephalopathy, Hepatic K72.90 Airway Stenosis J98.8 Esophageal Varices w/ bleeding I85.00 Cardiomyopathy, NOS, Idiopathic I42.8 Esophageal Varices W/O bleeding I85.01 Complication Heart Transplant - T86.21 Hepatitis A, Viral w/p hepatic coma B15.9 Complication Lung Transplant -T86.810 Hepatitis B, Chronic B18.1 COPD J44.9 Hepatitis B, Acute B16.9 Failure, Congestive Heart I50.9 Hepatitis C, Acute w/o hepatic coma B17.10 Fibrosis, Cystic E84.9 Hepatitis C, Chronic B18.2 Fibrosis, Pulmonary I84.10 Hepatosplenomegaly R16.2 Fibrosis, Idiopathic J84.112 Jaundice, not newborn -

Obscure Gastrointestinal Bleeding in Cirrhosis: Work-Up and Management
Current Hepatology Reports (2019) 18:81–86 https://doi.org/10.1007/s11901-019-00452-6 MANAGEMENT OF CIRRHOTIC PATIENT (A CARDENAS AND P TANDON, SECTION EDITORS) Obscure Gastrointestinal Bleeding in Cirrhosis: Work-up and Management Sergio Zepeda-Gómez1 & Brendan Halloran1 Published online: 12 February 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Obscure gastrointestinal bleeding (OGIB) in patients with cirrhosis can be a diagnostic and therapeutic challenge. Recent advances in the approach and management of this group of patients can help to identify the source of bleeding. While the work-up of patients with cirrhosis and OGIB is the same as with patients without cirrhosis, clinicians must be aware that there are conditions exclusive for patients with portal hypertension that can potentially cause OGIB. Recent Findings New endoscopic and imaging techniques are capable to identify sources of OGIB. Balloon-assisted enteroscopy (BAE) allows direct examination of the small-bowel mucosa and deliver specific endoscopic therapy. Conditions such as ectopic varices and portal hypertensive enteropathy are better characterized with the improvement in visualization by these techniques. New algorithms in the approach and management of these patients have been proposed. Summary There are new strategies for the approach and management of patients with cirrhosis and OGIB due to new develop- ments in endoscopic techniques for direct visualization of the small bowel along with the capability of endoscopic treatment for different types of lesions. Patients with cirrhosis may present with OGIB secondary to conditions associated with portal hypertension. Keywords Obscure gastrointestinal bleeding . Cirrhosis . Portal hypertension .