Esophageal Varices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Audit on Management of Hematemesis in Children Admitted to Pediatric Gastroenterology and Hepatology Unit of Assiut
Med. J. Cairo Univ., Vol. 86, No. 8, December: 4531-4536, 2018 www.medicaljournalofcairouniversity.net Clinical Audit on Management of Hematemesis in Children Admitted to Pediatric Gastroenterology and Hepatology Unit of Assiut University Children Hospital ESRAA T. AHMED, M.Sc.; FATMA A. ALI, M.D. and NAGLA H. ABU FADDAN, M.D. The Department of Pediatrics, Faculty of Medicine, Assiut University, Assiut, Egypt Abstract Hematemesis: Indicates that the bleeding origin is above the Treitz angle, i.e., that it constitutes an Background: Hematemesis is an uncommon but potentially Upper Gastrointestinal Bleeding (UGIB) [3] . serious and life-threatening clinical condition in children. It indicates that the bleeding origin is above the Treitz angle, The etiology of upper GI bleeding varies by i.e., that it constitutes an Upper Gastrointestinal Bleeding (UGIB). age. The pathophysiology of upper GI bleeding is related to the source of the bleeding. Most clinically Aim of Study: To assess for how much the adopted proto- significant causes of upper GI bleeds are associated cols of management of children with upper gastrointestinal bleeding were applied at Gastroenterology & Hepatology Unit with ulcers, erosive esophagitis, gastritis, varices, of Assiut University Children Hospital. and/or Mallory-Weiss tears. While Physiologic Patients and Methods: This study is a an audit on man- stress, NSAIDs such as aspirin and ibuprofen, and agement of children with upper gastrointestinal bleeding infection with Helicobacter pylori are few of the admitted to pediatric Gastroenterology and Hepatology Unit, factors contributing to the imbalance leading to Assiut University Children Hospital during the period from ulcers and erosions in the GI tract [4] . -

General Signs and Symptoms of Abdominal Diseases
General signs and symptoms of abdominal diseases Dr. Förhécz Zsolt Semmelweis University 3rd Department of Internal Medicine Faculty of Medicine, 3rd Year 2018/2019 1st Semester • For descriptive purposes, the abdomen is divided by imaginary lines crossing at the umbilicus, forming the right upper, right lower, left upper, and left lower quadrants. • Another system divides the abdomen into nine sections. Terms for three of them are commonly used: epigastric, umbilical, and hypogastric, or suprapubic Common or Concerning Symptoms • Indigestion or anorexia • Nausea, vomiting, or hematemesis • Abdominal pain • Dysphagia and/or odynophagia • Change in bowel function • Constipation or diarrhea • Jaundice “How is your appetite?” • Anorexia, nausea, vomiting in many gastrointestinal disorders; and – also in pregnancy, – diabetic ketoacidosis, – adrenal insufficiency, – hypercalcemia, – uremia, – liver disease, – emotional states, – adverse drug reactions – Induced but without nausea in anorexia/ bulimia. • Anorexia is a loss or lack of appetite. • Some patients may not actually vomit but raise esophageal or gastric contents in the absence of nausea or retching, called regurgitation. – in esophageal narrowing from stricture or cancer; also with incompetent gastroesophageal sphincter • Ask about any vomitus or regurgitated material and inspect it yourself if possible!!!! – What color is it? – What does the vomitus smell like? – How much has there been? – Ask specifically if it contains any blood and try to determine how much? • Fecal odor – in small bowel obstruction – or gastrocolic fistula • Gastric juice is clear or mucoid. Small amounts of yellowish or greenish bile are common and have no special significance. • Brownish or blackish vomitus with a “coffee- grounds” appearance suggests blood altered by gastric acid. -

Hemosuccus Pancreaticus: a Rare Cause of Upper Gastrointestinal Bleeding During Pregnancy Rani Akhil Bhat,1 Vani Ramkumar,1 K
Hemosuccus Pancreaticus: A Rare Cause Of Upper Gastrointestinal Bleeding During Pregnancy Rani Akhil Bhat,1 Vani Ramkumar,1 K. Akhil Krishnanand Bhat, 2 Rajgopal Shenoy2 Abstract Upper gastrointestinal bleeding is most commonly caused by From the 1Department of Department of Obstetrics and Gynaecology, Oman Medical 2 lesions in the esophagus, stomach or duodenum. Bleeding which College, Sohar, Sultanate of Oman, Department of Surgery, Oman Medical College, Sohar, Sultanate of Oma. originates from the pancreatic duct is known as hemosuccus pancreaticus. Only a few scattered case reports of hemosuccus Received: 06 Nov 2009 pancreaticus during pregnancy have been recorded in literature. Accepted: 31 Dec 2009 This is a case of a primigravida with 37 weeks of gestation Address correspondence and reprint request to: Dr. Rani A. Bhat,Department of with hemosuccus pancreaticus and silent chronic pancreatitis. Obstetrics and Gynaecology, Oman Medical College, P. O. Box 391, P. C. 321, Al- Evaluating pregnant women with upper gastrointestinal Tareef, Sohar, Sultanate of Oman. bleeding differs from that of non pregnant women as diagnostic E-mail: [email protected] modalities using radiation cannot be used. Therefore, Esophagogastroduodenoscopy should be performed at the time of active bleeding to diagnose hemosuccus pancreaticus. Bhat RA, et al. OMJ. 25 (2010); doi:10.5001/omj.2010.21 Introduction examination showed a combination of dark red blood and melena. Laboratory investigations revealed hemoglobin of 6.3 grams/dL, Hemosuccus pancreaticus is the term used to describe the liver function tests, serum amylase, glucose and prothrombin time syndrome of gastrointestinal bleeding into the pancreatic duct were within the normal range. -

Congestive Cholecystopathy; a Frequent Sonographic Sign of Evolving Esophageal Varices in Cirrhotics
Congestive Cholecystopathy; A Frequent Sonographic Sign of Evolving Esophageal Varices in Cirrhotics KHALID REHMAN YOUSAF, MIAN SAJID NISAR*, SALMAN ATIQ, AZHAR HUSSAIN*, AMNA RIZVI*, M. ISMAIL KHALID YOUSAF, ZAHID MANSOOR. Departments of Radiology and * Medicine, Omer Hospital, Lahore, Pakistan. Correspondence to Dr. Khalid Rehman Yousaf, Radiologist, New Radiology Department, SIMS/ S.H.L. Cell 923009458404 Email: [email protected] ABSTRACT Background: Gallbladder wall congestion (congestive cholecystopathy) is frequent sonographic feature demonstrated in patients with chronic liver disease. Hypoalbuminemia is still considered as a most probable cause of gallbladder wall thickness in cirrhotics. Objective: To demonstrate and establish congestive cholecystopathy as a consistent sonographic sign of developing portal hypertension and its association with evolving esophageal varices in Child’s class B (compensated) and C (decompensated) cirrhotic patients. Methodology: This cross-sectional study was conducted in Department of Radiology in collaboration with Department of Medicine, Omer Hospital, Lahore, between September 2009 and January 2011. We included 103 randomly sampled cirrhotic patients (67 men, 36 women; age range 38-79 years) who were clinically categorized into Class B and Class C liver disease through modified Child Pugh Classification. Upper gastrointestinal video endoscopy was performed for assessment of esophageal varices in all patients according to Japanese Research Society. Gall bladder targeted transabdominal ultrasound was performed on gray scale as well as color Doppler. Gallbladder wall thickness (4mm as a reference upper normal limit), pattern of wall thickening (striated or non-striated) and flow in wall were evaluated. Results: Out of 103 patients, 57 (55.3%) cases were of Child’s B and 46 (44.7%) of Child’s C class. -

Editorial Has the Time Come for Cyanoacrylate Injection to Become the Standard-Of-Care for Gastric Varices?
Tropical Gastroenterology 2010;31(3):141–144 Editorial Has the time come for cyanoacrylate injection to become the standard-of-care for gastric varices? Radha K. Dhiman, Narendra Chowdhry, Yogesh K Chawla The prevalence of gastric varices varies between 5% and 33% among patients with portal Department of Hepatology, hypertension with a reported incidence of bleeding of about 25% in 2 years and with a higher Postgraduate Institute of Medical bleeding incidence for fundal varices.1 Risk factors for gastric variceal hemorrhage include the education Research (PGIMER), size of fundal varices [more with large varices (as >10 mm)], Child class (C>B>A), and endoscopic Chandigarh, India presence of variceal red spots (defined as localized reddish mucosal area or spots on the mucosal surface of a varix).2 Gastric varices bleed less commonly as compared to esophageal Correspondence: Dr. Radha K. Dhiman, varices (25% versus 64%, respectively) but they bleed more severely, require more blood E-mail: [email protected] transfusions and are associated with increased mortality.3,4 The approach to optimal treatment for gastric varices remains controversial due to a lack of large, randomized, controlled trials and no clear clinical consensus. The endoscopic treatment modalities depend to a large extent on an accurate categorization of gastric varices. This classification categorizes gastric varices on the basis of their location in the stomach and their relationship with esophageal varices.1,5 Gastroesophageal varices are associated with varices along -

Venous Complications of Pancreatitis: a Review Yashant Aswani, Priya Hira Department of Radiology, Seth GS Medical College and KEM Hospital, Mumbai, Maharashtra INDIA
JOP. J Pancreas (Online) 2015 Jan 31; 16(1):20-24 REVIEW ARTICLE Venous Complications of Pancreatitis: A Review Yashant Aswani, Priya Hira Department of Radiology, Seth GS Medical College and KEM Hospital, Mumbai, Maharashtra INDIA ABSTRACT Pancreatitis is notorious to cause vascular complications. While arterial complications include pseudoaneurysm formation with a propensity to bleed, unusual venous complications associated with pancreatitis have, however, been described. In this article, we review multitudinous venous complications in thevenous setting complications of pancreatitis can andbe quite propose myriad. a system Venous to involvementclassify pancreatitis in pancreatitis associated often venous presents complications. with thrombosis. From time to time case reports and series of INTRODUCTION THROMBOTIC COMPLICATIONS IN PANCREATITIS Venous thrombosis is the most common complication of pancreatitis mediators and digestive enzymes. Consequently, pancreatitis associated complicationsPancreatitis is cana systemic be myriad disease with vascularowing to complications release of inflammatory being a well known but infrequent phenomenon. These vascular complications affecting venous system. A surge in procoagulant inflammatory mediators, are seen in 25% patients suffering from pancreatitis and entail chronicstasis, vesselpancreatitis spasm, (CP) mass includes effects intimal from injury the surroundingdue to repeated inflamed acute pancreas causes thrombosis in acute pancreatitis [2] whereas etiology in of peripancreatic arteries. Venous complications are less commonly significant morbidity and mortality [1]. There is predominant affliction tiesinflammation, with pancreas chronic results inflammation in splenic veinwith involvement fibrosis, compressive in majority effectsof the of a pseudocyst or an enlarged inflamed pancreas [3]. Close anatomic complicationsreported and associatedare often withconfined pancreatitis to thrombosis have, however, of the been vein. described. Isolated 22% (Agrawal et al.) and 5.6% (Bernades et al.), respectively. -

Correlation of Portal Vein Size with Esophageal Varices Severity in Patients with Cirrhosis of Liver with Portal Hypertension
International Journal of Scientific and Research Publications, Volume 5, Issue 1, January 2015 1 ISSN 2250-3153 Correlation of Portal Vein Size with Esophageal Varices Severity in Patients with Cirrhosis of Liver with Portal Hypertension. Dr. K.V.L. Sudha Rani*, Dr. B. Sudarsi**, Dr. R. Siddeswari***, Dr. S. Manohar, **** * M.D., Asst. Prof. of Medicine ** M.D., Asst Prof. of Medicine *** M.D., Prof. of Medicine ****M.D., Prof. & HOD of Medicine. Abstract- This study was conducted to correlate the portal vein Complications of cirrhosis include portal hypertension, diameter measured by ultrasound to development of oesophageal spontaneous bacterial peritonitis, hepato renal syndrome, hepatic varices in patients with cirrhosis of liver with portal encephalopathy and hematological abnormalities. hypertension. Portal vein is formed by confluence of superior mesenteric 92 patients with cirrhosis admitted in OGH were selected for vein and splenic vein. Portal hypertension is defined as elevation the study USG was conducted in all patients to note portal vein of hepatic venous pressure gradient more than 5 mmHg. Portal size and splenic size. Upper GI endoscopy was done to detect hypertension is caused by. 1) Increased intra hepatic resistance to presence of varices with different grades. passage of blood flow through the liver due to cirrhosis and 2) In this study 65 patients had varices. Out of 65 patients 30 increased splanchnic blood flow secondary to vasodilation with had large varices (Grade III & IV) of 65 patients, 53 patients in splanchnic vascular bed. with varices have portal vein diameter > 13 mm patients, with Congestive splenomegaly is common in patients with portal small varices and those without varices portal vein diameter is < hypertension. -

Chapter 156: Upper Gastrointestinal Bleeding
8/23/2018 Principles and Practice of Hospital Medicine, 2e > Chapter 156: Upper Gastrointestinal Bleeding Stephen R. Rotman; John R. Saltzman INTRODUCTION Key Clinical Questions What is the timing and treatment of peptic ulcer disease? What are the factors in diagnosis and treatment of aortoenteric fistula? What treatments are available for each etiology of upper GI bleeding? What is the appropriate management and follow-up of variceal bleeding? How do you estimate the severity of bleeding so that you can triage appropriate patients to the ICU, medical floor, or observation unit? Which patients are more likely to rebleed and hence require continued observation in the hospital aer their bleeding has apparently stopped, and for how long? Upper gastrointestinal (GI) bleeding is responsible for over 300,000 hospitalizations per year in the United States. An additional 100,000 to 150,000 patients develop upper GI bleeding during hospitalizations. The annual cost of treating nonvariceal acute upper GI bleeding in the United States exceeds $7 billion. Upper GI bleeding is defined as a bleeding source in the GI tract proximal to the ligament of Treitz. The presentation varies depending on the nature and severity of bleeding and includes hematemesis, melena, hematochezia (in rapid upper GI bleeding), and anemia with heme-positive stools. Bleeding can be associated with changes in vital signs, including tachycardia and hypotension including orthostatic hypotension. Given the range of presentations, pinpointing the nature and severity of GI bleeding may be a challenging task. The natural history of nonvariceal upper GI bleeding is that 80% of patients will stop bleeding spontaneously and no further urgent intervention will be needed. -

Banana May Be Forbidden After Endoscopic Variceal Ligation: a Case Report
Case Report Banana may be forbidden after endoscopic variceal ligation: a case report Yingying Li1,2#, Xiaozhong Guo1#, Zhaohui Bai1,3, Xiaodong Shao1, Ran Wang1, Hongyu Li1, Xingshun Qi1 1Department of Gastroenterology, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), Shenyang 110840, China; 2Postgraduate College, Jinzhou Medical University, Jinzhou 121001, China; 3Postgraduate College, Shenyang Pharmaceutical University, Shenyang 110840, China #These authors contributed equally to this work. Correspondence to: Dr. Xingshun Qi, MD; Prof. Hongyu Li. General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), No. 83 Wenhua Road, Shenyang 110840, China. Email: [email protected]; [email protected]. Abstract: Acute variceal hemorrhage (AVH) is a devastating complication of liver cirrhosis. Endoscopic variceal ligation (EVL) is a useful endoscopic treatment for AVH with few complications. However, the issue regarding management of early re-bleeding after EVL still needs to be concerned. Furthermore, the dietary principle after EVL is unclear. There is no consensus regarding what food should be eaten after EVL. In this paper, we reported a patient who ate a banana after an EVL and then developed early re-bleeding episodes. Keywords: Endoscopic variceal ligation (EVL); portal hypertension; banana; re-bleeding; dietary Received: 02 November 2018; Accepted: 27 January 2019; Published: 20 February 2019. doi: 10.21037/tgh.2019.01.11 View this article at: http://dx.doi.org/10.21037/tgh.2019.01.11 Introduction Case presentation Acute variceal hemorrhage (AVH) is a lethal consequence On May 24, 2018, a 41-year-old male was admitted to of portal hypertension in liver cirrhosis patients (1,2). -

Overview of Esophageal and Gastric Varices
pISSN 2287-2728 eISSN 2287-285X https://doi.org/10.3350/cmh.2020.0022 Review Clinical and Molecular Hepatology 2020;26:444-460 Managing liver cirrhotic complications: Overview of esophageal and gastric varices Cosmas Rinaldi Adithya Lesmana1,2, Monica Raharjo1, and Rino A. Gani1 1Division of Hepatobiliary, Department of Internal Medicine, Dr. Cipto Mangunkusumo National General Hospital, Medical Faculty Universitas Indonesia, Jakarta; 2Digestive Disease & GI Oncology Centre, Medistra Hospital, Jakarta, Indonesia Managing liver cirrhosis in clinical practice is still a challenging problem as its progression is associated with serious complications, such as variceal bleeding that may increase mortality. Portal hypertension (PH) is the main key for the development of liver cirrhosis complications. Portal pressure above 10 mmHg, termed as clinically significant portal hypertension, is associated with formation of varices; meanwhile, portal pressure above 12 mmHg is associated with variceal bleeding. Hepatic vein pressure gradient measurement and esophagogastroduodenoscopy remain the gold standard for assessing portal pressure and detecting varices. Recently, non-invasive methods have been studied for evaluation of portal pressure and varices detection in liver cirrhotic patients. Various guidelines have been published for clinicians’ guidance in the management of esophagogastric varices which aims to prevent development of varices, acute variceal bleeding, and variceal rebleeding. This writing provides a comprehensive review on development of PH and varices in liver cirrhosis patients and its management based on current international guidelines and real experience in Indonesia. (Clin Mol Hepatol 2020;26:444-460) Keywords: Liver cirrhosis; Hypertension, Portal; Esophageal and gastric varices INTRODUCTION tes, hepatic encephalopathy, coagulation dysfunction, hepatorenal syndrome, and even cardiac and pulmonary complications. -
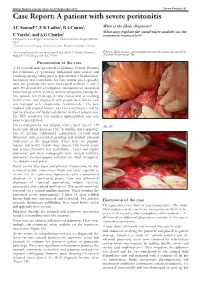
Case Report: a Patient with Severe Peritonitis
Malawi Medical Journal; 25(3): 86-87 September 2013 Severe Peritonitis 86 Case Report: A patient with severe peritonitis J C Samuel1*, E K Ludzu2, B A Cairns1, What is the likely diagnosis? 2 1 What may explain the small white nodules on the C Varela , and A G Charles transverse mesocolon? 1 Department of Surgery, University of North Carolina, Chapel Hill NC USA 2 Department of Surgery, Kamuzu Central Hospital, Lilongwe Malawi Corresponding author: [email protected] 4011 Burnett Womack Figure1. Intraoperative photograph showing the transverse mesolon Bldg CB 7228, Chapel Hill NC 27599 (1a) and the pancreas (1b). Presentation of the case A 42 year-old male presented to Kamuzu Central Hospital for evaluation of worsening abdominal pain, nausea and vomiting starting 3 days prior to presentation. On admission, his history was remarkable for four similar prior episodes over the previous five years that lasted between 3 and 5 days. He denied any constipation, obstipation or associated hematemesis, fevers, chills or urinary symptoms. During the first episode five years ago, he was evaluated at an outlying health centre and diagnosed with peptic ulcer disease and was managed with omeprazole intermittently . His past medical and surgical history was non contributory and he had no allergies and he denied alcohol intake or tobacco use. His HIV serostatus was negative approximately one year prior to presentation. On examination he was afebrile, with a heart rate of 120 (Fig 1B) beats/min, blood pressure 135/78 mmHg and respiratory rate of 22/min. Abdominal examination revealed mild distension with generalized guarding and marked rebound tenderness in the epigastrium. -

Esophageal Varices
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Crossref Hindawi Publishing Corporation Case Reports in Critical Care Volume 2016, Article ID 2370109, 4 pages http://dx.doi.org/10.1155/2016/2370109 Case Report A Rare but Reversible Cause of Hematemesis: (Downhill) Esophageal Varices Lam-Phuong Nguyen,1,2,3 Narin Sriratanaviriyakul,1,2,3 and Christian Sandrock1,2,3 1 Division of Pulmonary, Critical Care, and Sleep Medicine, University of California, Davis, Suite #3400, 4150 V Street, Sacramento, CA 95817, USA 2Department of Internal Medicine, University of California, Davis, Sacramento, USA 3VA Northern California Health Care System, Mather, USA Correspondence should be addressed to Lam-Phuong Nguyen; [email protected] Received 12 December 2015; Accepted 1 February 2016 Academic Editor: Kurt Lenz Copyright © 2016 Lam-Phuong Nguyen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. “Downhill” varices are a rare cause of acute upper gastrointestinal bleeding and are generally due to obstruction of the superior vena cava (SVC). Often these cases of “downhill” varices are missed diagnoses as portal hypertension but fail to improve with medical treatment to reduce portal pressure. We report a similar case where recurrent variceal bleeding was initially diagnosed as portal hypertension but later found to have SVC thrombosis presenting with recurrent hematemesis. A 39-year-old female with history of end-stage renal disease presented with recurrent hematemesis. Esophagogastroduodenoscopy (EGD) revealed multiple varices.