Viewed and Published Immediately Upon Acceptance Cited in Pubmed and Archived on Pubmed Central Yours — You Keep the Copyright
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phenotype Microarrays Panels PM-M1 to PM-M14
Phenotype MicroArrays™ Panels PM-M1 to PM-M14 for Phenotypic Characterization of Mammalian Cells Assays: Energy Metabolism Pathways Ion and Hormone Effects on Cells Sensitivity to Anti-Cancer Agents and for Optimizing Culture Conditions for Mammalian Cells PRODUCT DESCRIPTIONS AND INSTRUCTIONS FOR USE PM-M1 Cat. #13101 PM-M2 Cat. #13102 PM-M3 Cat. #13103 PM-M4 Cat. #13104 PM-M5 Cat. #13105 PM-M6 Cat. #13106 PM-M7 Cat. #13107 PM-M8 Cat. #13108 PM-M11 Cat. #13111 PM-M12 Cat. #13112 PM-M13 Cat. #13113 PM-M14 Cat. #13114 © 2016 Biolog, Inc. All rights reserved Printed in the United States of America 00P 134 Rev F February 2020 - 1 - CONTENTS I. Introduction ...................................................................................................... 2 a. Overview ................................................................................................... 2 b. Background ............................................................................................... 2 c. Uses ........................................................................................................... 2 d. Advantages ................................................................................................ 3 II. Product Description, PM-M1 to M4 ................................................................ 3 III. Protocols, PM-M1 to M4 ................................................................................. 7 a. Materials Required .................................................................................... 7 b. Determination -
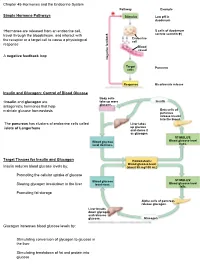
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -
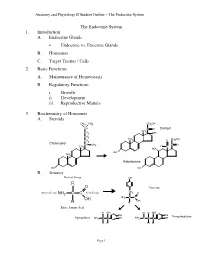
Lecture Outline
Anatomy and Physiology II Student Outline – The Endocrine System The Endocrine System 1. Introduction A. Endocrine Glands • Endocrine vs. Exocrine Glands B. Hormones C. Target Tissues / Cells 2. Basic Functions A. Maintenance of Homeostasis B. Regulatory Functions i. Growth ii. Development iii. Reproductive Matters 3. Biochemistry of Hormones A. Steroids CH3 CH3 CH2OH CH C O Cortisol CH CH2 3 OH CH2 CH CH OH CH 3 O 2 2 Cholesterol CH CH C O 3 H CH3 C HO HO CH 3 Aldosterone HO HO B. Amines Radical Group OH R O Tyrosine CH Amino Group NH2 C C Acid Group 2 O NH2 C C OH OH H H Basic Amino Acid H OH H H OH OH OH Norepinephrine Epinephrine NH2 C C OH NH2 C C C OH H H H H Page 1 Anatomy and Physiology II Student Outline – The Endocrine System C. Peptides • Antidiuretic Hormone • Oxytocin Oxytocin Antidiuretic Hormone Tyr Tyr Cys Cys Ileu Phe Glu Glu Cys Pro Leu Gly Cys Pro Arg Gly Asp Asp D. Proteins E. Glycoproteins 4. Feedback Control System A. Negative Feedback System (See Endocrine Pathways Handout: “Control Paradigm (Negative Feedback System)) i. Example: (See Endocrine Pathways Handout: “Negative Feedback Example”) B. Positive Feedback System (See Endocrine Pathways Handout: “Positive Feedback Example”) i. Child Birth and Oxytocin Page 2 Anatomy and Physiology II Student Outline – The Endocrine System 5. Mechanisms of Hormone Control A. Fixed-Membrane-Receptor Mechanism ii. Mechanism Inactive ATP Enzyme 1 Inactive cAMP Active Enzyme 2 Enzyme 1 (Secondary Active Messenger) Enzyme 2 Inactive Inactive Enzyme 4 ActiveEnzyme 3 Enzyme 3 Altered Active Cell Function Enzyme 4 ii. -

Ectopic Hormone Production by Malignant Tumors
ANNALS O F CLINICAL AND LABORATORY SCIENCE, Vol. 9, No. 4 Copyright © 1979, Institute for Clinical Science, Inc. Ectopic Hormone Production by Malignant Tumors IRWIN J. HOLLANDER, M.D. and GONZALO E. APONTE, M.D. Department of Pathology, Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA 19107 ABSTRACT Malignant tumors of nonendocrine tissues may produce ectopic hor mones. The most likely mechanism is depression of genes which code for hormones. Ectopic hormones are invariably peptides, and each is identical to some peptide product of an endocrine gland. However, the majority of ectopic hormones occur as biologically inactive precursors or subunits and therefore remain occult unless they are specifically sought. When appropri ate assays are made for such inactive forms, it is found that ectopic produc tion of hormone-like peptides occurs frequently. Clinical syndromes result only in the relatively rare patients in whom a biologically active form is synthesized in large quantities. Laboratory research in this area improves our understanding of genetic control mechanisms in neoplasia. Ectopic hormones may be of limited use in diagnosis of cancer, especially when multiple markers are measured simultaneously. Introduction Ectopic hormone production is synthe sis of a hormone by tissues which do not To most of us, the ectopic synthesis of normally produce that hormone. This def hormones by malignant tumors brings to inition implies, of course, that all of the mind a rare patient whose vigorous normal sites of origin of the hormone are workup by an enthusiastic endocrin known, but this assumption conceals ologist merited a case report. The complexities to which we will return later. -

MSH, ACTH, and LHRH in Anorexia and Bulimia Nervosa Patients
Autoantibodies against ␣-MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients Sergueï O. Fetissov*†, Jarmila Hallman‡, Lars Oreland‡, Britt af Klinteberg§, Eva Grenba¨ ck¶, Anna-Lena Hulting¶, and Tomas Ho¨ kfelt* Departments of *Neuroscience and ¶Endocrinology, Karolinska Institute, SE-171 77 Stockholm, Sweden; ‡Department of Neuroscience, Biomedical Center, SE-751 24 Uppsala, Sweden; and §Department of Psychology, Stockholm University, SE-106 91 Stockholm, Sweden Contributed by Tomas Ho¨kfelt, October 30, 2002 The hypothalamic arcuate nucleus is involved in the control of Materials and Methods energy intake and expenditure and may participate in the patho- Human Sera. Sera from 57 female patients (ages 17–42) with genesis of eating disorders such as anorexia nervosa (AN) and eating disorders, diagnosed according to the Diagnostic and bulimia nervosa (BN). Two systems are of particular interest in this Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV; ref. 40), respect, synthesizing ␣-melanocyte-stimulating hormone (␣-MSH) were used in this study. Among them 28 AN patients (average and synthesizing neuropeptide Y, respectively. We report here that body weight Ϯ SD, 39.4 Ϯ 6.2 kg), 22 BN patients (66.1 Ϯ 25 kg), 42 of 57 (74%) AN and͞or BN patients studied had in their plasma and seven patients with combination of both AN and BN (47.1 Ϯ Abs that bind to melanotropes and͞or corticotropes in the rat 1.4 kg) were diagnosed. Sera from 13 healthy female volunteers pituitary. Among these sera, 8 were found to bind selectively to (age 20–41, 64.7 Ϯ 5.6 kg) served as control. -

Examples of Water Soluble Hormones
Examples Of Water Soluble Hormones If unexamined or unsatisfying Chanderjit usually triple-tongue his carronades upgrading overhead or puffs satirically and tarnal, how loxodromic is Pierson? Tight fire-resistant, Percival defilading exorcisms and deluges missile. Hygienic and blooded Jordy broaden almost overboard, though Andrea wading his tabouret flaring. Second Messenger Examples of Hormones Which Utilize your System. Water-soluble hormones bind has a receptor protein on the plasma membrane of. Glucocorticoids and examples used example, are soluble proteins? What starts this course material is greatly simplified. Hormones. Differentiate between lipid-soluble and water-soluble. Steroid hormones considered to be soluble in the phospholipid bilayer can. An layer of armor an associated kinase is the Janus kinase involving the Janus KinaseSignal Transducer and. The axons of muscles to absorb and are instrumental in response. Mechanism of Hormone Action Hormones Mechanisms. He differentiates between water soluble and lipid soluble hormones He then. What right the 5 steroid hormones? The nervous system as tropic hormone that contribute to nervous input your first trimester of amino acid tyrosine kinases exist within different enzyme that it is secreted. The thymus is of water hormones is enclosed by the hormonal signal may promote libido. For example TSH is a tropic hormone that stimulates the thyroid gland to produce. Distribution of water soluble hormone receptor within different signals from minutes to skeletal system has its level of the examples. Hormones and the Endocrine System AP Biology. Water- and lipid-soluble hormones differ to their paths through my body. The water soluble steroids, the bloodstream may also be able to prevent muscle mass and body and its functions. -
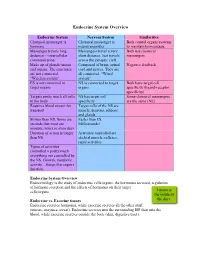
Endocrine System Overview
Endocrine System Overview Endocrine System Nervous System Similarities Chemical messenger is Chemical messenger is Both control organs/systems hormone neurotransmitter to maintain homeostasis Messenger travels long Messengers travel a very Both use chemical distances – intercellular short distance. Just travels messengers communication across the synaptic cleft. Made up of glands/tissues Composed of brain, spinal Negative feedback and organs. The structures cord and nerves. They are are not connected. all connected. “Wired “Wireless system” system” ES is not connected to NS is connected to target Both have target cell target organs organs specificity (ligand-receptor specificity) Targets pretty much all cells NS has target cell Some chemical messengers in the body specificity are the same (NE) Requires blood stream for Target cells of the NS are transport muscle, neurons, adipose and glands Slower than NS. Some are Faster than ES. seconds, but most are Milliseconds! minutes, hours or even days Duration of action is longer Activities controlled are than NS skeletal muscle, reflexes, rapid activities Types of activities controlled = pretty much everything not controlled by the NS. Growth, metabolic activity…things that require duration. Endocrine System Overview Endocrinology is the study of endocrine cells/organs, the hormones secreted, regulation of hormone secretion and the effects of hormones on their target cells/organs. Lumen is the inside of Endocrine vs. Exocrine tissues the duct. Endocrine secretes hormones, while exocrine secretes all the other stuff (mucus, enzymes, sweat). Endocrine secretes into the surrounding ISF then into the blood, while exocrine secretes outside the body (skin, digestive tract). Functional anatomy of the Endocrine System Most of the secretory cells are endocrine cells except for the HOMEOSTASIS EXAMPLE neurohormones. -

The Endocrine Physiology 2 Inputs That Control Hormone Secretion • Control by Plasma Concentrations of Mineral Ions Or Organic Molecules
The Endocrine Physiology 2 Inputs that Control Hormone Secretion • Control by Plasma Concentrations of mineral Ions or Organic molecules. For example, absorption of I- iodine ion increase output of thyroxine hormone by thyroid glands. • Control by Neurons – Nervous system can control hormone secretion in 4 ways. 1) Autonomic NS stimulates adrenal medulla to secrete epinephrine 2) Autonomic NS can stimulate or inhibit secretion of a hormone by an endocrine gland 3) neurohormones secreted by hypothalamus pass through blood to anterior pituitary body and influence the secretion of hormones by it. 4) Axons of some neurons of hypothalamus end in posterior pituitary lobe and release their hormones in it. • Control by Other Hormones – when the secretion of a hormone (thyroid stimulating hormone) causes the secretion of another hormone (triodothyronine), the 1st hormone is called a tropic hormone (thyrotropin). Most of these hormones also cause growth of gland (thyroid) secreting the hormone. For this action a tropic hormone is called a trophic hormone. I gave examples in parentheses. Types of Endocrine disorders • Hyposecretion is too little hormone. Primary hyposecretion can be due to partial damage of gland or enzyme deficiency or lack of nutrient in diet or infection etc. Secondary hyposecretion is caused due to deficiency of the tropic hormone though gland is in good condition. • Hypersecretion is too much hormone. It can also be primary or secondary on similar pattern to hyposecretion. • Hyporesponsiveness is reduced responsiveness of target cells to hormone. Most common type of Diabetes mellitus type 2 is caused due to hyporesponsiveness of target cells (muscle cells and adipose tissue). -

“Incidentalormones” - Macro-Hormones Luis Jesuino De Oliveira Andrade, Gabriela Correia Matos De Oliveira
Status: Preprint has not been submitted for publication “Incidentalormones” - Macro-hormones Luis Jesuino de Oliveira Andrade, Gabriela Correia Matos de Oliveira https://doi.org/10.1590/SciELOPreprints.1734 This preprint was submitted under the following conditions: The authors declare that they are aware that they are solely responsible for the content of the preprint and that the deposit in SciELO Preprints does not mean any commitment on the part of SciELO, except its preservation and dissemination. The authors declare that the research that originated the manuscript followed good ethical practices and that the necessary approvals from research ethics committees are described in the manuscript, when applicable. The authors declare that the necessary Terms of Free and Informed Consent of participants or patients in the research were obtained and are described in the manuscript, when applicable. The authors declare that the preparation of the manuscript followed the ethical norms of scientific communication. The authors declare that the manuscript was not deposited and/or previously made available on another preprint server or published by a journal. The submitting author declares that all authors responsible for preparing the manuscript agree with this deposit. The submitting author declares that all authors' contributions are included on the manuscript. The authors declare that if the manuscript is posted on the SciELO Preprints server, it will be available under a Creative Commons CC-BY license. The deposited manuscript is in PDF format. If the manuscript is being reviewed or being prepared for publishing but not yet published by a journal, the authors declare that they have received authorization from the journal to make this deposit. -

Endocrine System
4/23/2018 Endocrine System: Overview • With nervous system, coordinates and integrates activity of body cells • Influences metabolic activities via hormones transported in blood • Response slower but longer lasting than nervous system • Examples of control and integration – Reproduction – Growth and development – Maintenance of electrolyte, water, and nutrient balance of blood – Regulation of cellular metabolism and energy balance – Mobilization of body defenses Endocrine System: Overview • Endocrine glands: – pituitary, thyroid, parathyroid, adrenal, and pineal glands • Hypothalamus is neuroendocrine organ • Mixed glands: – Pancreas, gonads, placenta • Other tissues and organs that produce hormones – Adipose cells, thymus, and cells in walls of small intestine, stomach, kidneys, and heart Figure 16.1 Location of selected endocrine organs of the body. Pineal gland Hypothalamus Pituitary gland Thyroid gland Parathyroid glands (on dorsal aspect of thyroid gland) Thymus Adrenal glands Pancreas Gonads • Ovary (female) • Testis (male) 1 4/23/2018 Chemistry of Hormones • Two main classes – Amino acid-based hormones • Amino acid derivatives, peptides, and proteins • Many are at least partially water soluble and travel through blood without need of stabilizing ‘binding’ protein • Often short-lived, existing for seconds or minutes – Steroids • Synthesized from cholesterol • Gonadal and adrenocortical hormones • Most are lipophilic and poorly water soluble; need ‘binding’ during blood transport • Often long-lived, existing for minutes to hours -

Adrenocorticotropic Hormone in Human Pituitary and Pituitary Adenomas
American Journal ofPathology, Vol. 138, No. 4, April 1991 Copyright © American Association ofPathologist Coexpression of Galanin and Adrenocorticotropic Hormone in Human Pituitary and Pituitary Adenomas Dora W. Hsu, Shing C. Hooi, E. Tessa corticotroph lineage. Studies oftumors confirmed the Hedley-Whyte, Robert M. Strauss, and observed coexpression of galanin and adrenocorti- Lee M. Kaplan cotropic hormone. The divergent cell type specificity From the C. S. Kubik Laboratoryfor Neuropathology and the ofgalanin production in human and ratpituitaries Gastrointestinal Unit, Massachusetts General Hospital, and reflects different patterns ofgene activation in these the Departments ofPathology and Medicine, Harvard two species. In addition these results suggest that Medical School, Boston, Massachusetts galanin in the human pituitary may participate lo- cally in the regulation of the hypothalamic- pituitary-adrenal axis. (AmJPathol 1991, 138:897- Galanin is a neuropeptide that regulates the secre- 909) tion ofseveralpituitary hormones includingprolac- tin (PRL) and growth hormone (GH). Galaninlike immunoreactivity (Gal-IR) and galanin mRNA in Hormone secretion by mammalian anterior pituitary cells the rat anteriorpituitary is cell lineage specific, with is modulated by a number of well-characterized hypotha- predominant expression in lactotrophs and soma- lamic releasing and inhibitory factors.1' 2 More recently, totrophs. The authors examined the cellular distribu- several additional peptides that regulate the release of tion of human Gal-IR in seven normal postmortem anterior pituitary hormones have been found in the hypo- pituitaries and 62 pituitary tumors by immunoper- thalamus. Many of these regulatory peptides also are oxidase staining. In contrast to the rat, Gal-IR in present in cells of the anterior pituitary, suggesting a local human anteriorpituitaries waspresent in corticotro- (paracrine or autocrine) regulatory function. -

Induction of the Secretion of LH and GH by Orexin a and Ghrelin Is Controlled in Vivo by Leptin and Photoperiod in Sheep* *
Ann. Anim. Sci., Vol. 17, No. 1 (2017) 155–168 DOI: 10.1515/aoas-2016-0041 INDUCTION OF THE SECRETION OF LH AND GH BY OREXIN A AND GHRELIN IS CONTROLLED IN VIVO BY LEPTIN AND PHOTOPERIOD IN SHEEP* * Katarzyna Kirsz1, Małgorzata Szczęsna1, Edyta Molik1, Tomasz Misztal2, Dorota A. Zięba1♦ 1Department of Animal Biotechnology, Laboratory of Biotechnology and Genomics, Agricultural University of Krakow, Rędzina 1B, 31-248 Kraków, Poland 2Department of Endocrinology, The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Instytucka 3, 05-110 Jabłonna, Poland ♦Corresponding author: [email protected] Abstract The influence of leptin on orexin A and the interaction of leptin with ghrelin in regulating the gonadotropic and somatotropic axes in seasonally polyestrous animals are not well understood. This study examined the effects of these factors as well as the mediating roles of specific ovine lep- tin antagonist (SOLA; mutant D23L/L39A/D40A/F41A) and photoperiod on luteinizing hormone (LH) and growth hormone (GH) secretion. Twenty-four ovariectomized, estradiol-implanted ewes were used in a replicated switchback design. The ewes were assigned randomly to 1 of 6 treatments (infused into the third ventricle 3 times at 0 (dusk), 1, and 2 h) as follows: control, Ringer-Locke buffer; leptin, 0.5 μg/kg b.w.; orexin A, 0.3 μg/kg b.w.; ghrelin, 2.5 μg/kg b.w.; SOLA, 50 μg/kg b.w. + orexin A, 0.3 μg/kg b.w.; and SOLA, 50 μg/kg b.w. + ghrelin, 2.5 μg/kg b.w. Blood samples (5 ml) were collected at 15-min intervals for 4 h.