Non-Opioid Peptides for Analgesia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phenotype Microarrays Panels PM-M1 to PM-M14
Phenotype MicroArrays™ Panels PM-M1 to PM-M14 for Phenotypic Characterization of Mammalian Cells Assays: Energy Metabolism Pathways Ion and Hormone Effects on Cells Sensitivity to Anti-Cancer Agents and for Optimizing Culture Conditions for Mammalian Cells PRODUCT DESCRIPTIONS AND INSTRUCTIONS FOR USE PM-M1 Cat. #13101 PM-M2 Cat. #13102 PM-M3 Cat. #13103 PM-M4 Cat. #13104 PM-M5 Cat. #13105 PM-M6 Cat. #13106 PM-M7 Cat. #13107 PM-M8 Cat. #13108 PM-M11 Cat. #13111 PM-M12 Cat. #13112 PM-M13 Cat. #13113 PM-M14 Cat. #13114 © 2016 Biolog, Inc. All rights reserved Printed in the United States of America 00P 134 Rev F February 2020 - 1 - CONTENTS I. Introduction ...................................................................................................... 2 a. Overview ................................................................................................... 2 b. Background ............................................................................................... 2 c. Uses ........................................................................................................... 2 d. Advantages ................................................................................................ 3 II. Product Description, PM-M1 to M4 ................................................................ 3 III. Protocols, PM-M1 to M4 ................................................................................. 7 a. Materials Required .................................................................................... 7 b. Determination -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -
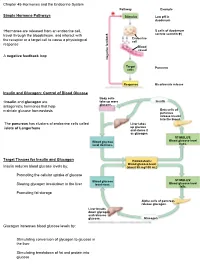
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -

Cholecystokinin Octapeptide Antagonized Opioid Analgesia Mediated by /T- and R- but Not Cs-Receptors in the Spinal Cord of the Rat
Brain Research, 523 (1990) 5-10 5 Elsevier BRES 15696 Cholecystokinin octapeptide antagonized opioid analgesia mediated by /t- and r- but not cS-receptors in the spinal cord of the rat Xiao-Jing Wang, Xiao-Hong Wang and Ji-Sheng Han Department of Physiology, Beijing Medical University, Beijing (People's Republic of China) (Accepted 23 January 1990) Key words: Cholecystokinin octapeptide; (N-MePhea,o-Pro4) Morphiceptin; (N-MeTyrl,N-MeArg7,D-Leu 8) Dynorphin(1-8) ethylamide; (D-Pen 2'5) Enkephalin; Proglumide; Intrathecal injection; Opioid analgesia; Antiopioid effect Intrathecal (ith) injection of cholecystokinin octapeptide (CCK-8) to the rat with single dose of 4 or 40 ng, or successive doses from 0.1 to 1/ag at 10 rain intervals produced neither analgesia nor hyperalgesia. However, the analgesia produced by ith injection of PL017, a specific /~-reeeptor agonist or 66A-078, a specific r-receptor agonist could be markedly antagonized by CCK-8 at a dose as small as 4 ng. In contrast, analgesia produced by ith injection of 6-agonist DPDPE could not be blocked by CCK-8 even at a dose as high as 40 ng. Since the effect of CCK-8 could be totally reversed by the CCK receptor antagonist proglumide, this effect is most probably mediated by CCK receptors. INTRODUCTION MATERIALS AND METHODS Cholecystokinin octapeptide (CCK-8) has been known Surgical procedures and intrathecal injection of drugs Male Wistar rats weighing 200-250 g were anesthetized with as a neuropeptide of abundant and wide distribution in chlorohydrate (0.4 g/kg, i.p.). PE-10 polyethiene catheter of 7.5 cm CNS 1 with some important physiological functions in- long was implanted through the atlanto-occipital membrane down to cluding the anti-opioid effect4'9. -
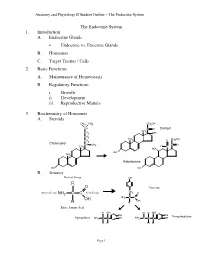
Lecture Outline
Anatomy and Physiology II Student Outline – The Endocrine System The Endocrine System 1. Introduction A. Endocrine Glands • Endocrine vs. Exocrine Glands B. Hormones C. Target Tissues / Cells 2. Basic Functions A. Maintenance of Homeostasis B. Regulatory Functions i. Growth ii. Development iii. Reproductive Matters 3. Biochemistry of Hormones A. Steroids CH3 CH3 CH2OH CH C O Cortisol CH CH2 3 OH CH2 CH CH OH CH 3 O 2 2 Cholesterol CH CH C O 3 H CH3 C HO HO CH 3 Aldosterone HO HO B. Amines Radical Group OH R O Tyrosine CH Amino Group NH2 C C Acid Group 2 O NH2 C C OH OH H H Basic Amino Acid H OH H H OH OH OH Norepinephrine Epinephrine NH2 C C OH NH2 C C C OH H H H H Page 1 Anatomy and Physiology II Student Outline – The Endocrine System C. Peptides • Antidiuretic Hormone • Oxytocin Oxytocin Antidiuretic Hormone Tyr Tyr Cys Cys Ileu Phe Glu Glu Cys Pro Leu Gly Cys Pro Arg Gly Asp Asp D. Proteins E. Glycoproteins 4. Feedback Control System A. Negative Feedback System (See Endocrine Pathways Handout: “Control Paradigm (Negative Feedback System)) i. Example: (See Endocrine Pathways Handout: “Negative Feedback Example”) B. Positive Feedback System (See Endocrine Pathways Handout: “Positive Feedback Example”) i. Child Birth and Oxytocin Page 2 Anatomy and Physiology II Student Outline – The Endocrine System 5. Mechanisms of Hormone Control A. Fixed-Membrane-Receptor Mechanism ii. Mechanism Inactive ATP Enzyme 1 Inactive cAMP Active Enzyme 2 Enzyme 1 (Secondary Active Messenger) Enzyme 2 Inactive Inactive Enzyme 4 ActiveEnzyme 3 Enzyme 3 Altered Active Cell Function Enzyme 4 ii. -

Ectopic Hormone Production by Malignant Tumors
ANNALS O F CLINICAL AND LABORATORY SCIENCE, Vol. 9, No. 4 Copyright © 1979, Institute for Clinical Science, Inc. Ectopic Hormone Production by Malignant Tumors IRWIN J. HOLLANDER, M.D. and GONZALO E. APONTE, M.D. Department of Pathology, Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA 19107 ABSTRACT Malignant tumors of nonendocrine tissues may produce ectopic hor mones. The most likely mechanism is depression of genes which code for hormones. Ectopic hormones are invariably peptides, and each is identical to some peptide product of an endocrine gland. However, the majority of ectopic hormones occur as biologically inactive precursors or subunits and therefore remain occult unless they are specifically sought. When appropri ate assays are made for such inactive forms, it is found that ectopic produc tion of hormone-like peptides occurs frequently. Clinical syndromes result only in the relatively rare patients in whom a biologically active form is synthesized in large quantities. Laboratory research in this area improves our understanding of genetic control mechanisms in neoplasia. Ectopic hormones may be of limited use in diagnosis of cancer, especially when multiple markers are measured simultaneously. Introduction Ectopic hormone production is synthe sis of a hormone by tissues which do not To most of us, the ectopic synthesis of normally produce that hormone. This def hormones by malignant tumors brings to inition implies, of course, that all of the mind a rare patient whose vigorous normal sites of origin of the hormone are workup by an enthusiastic endocrin known, but this assumption conceals ologist merited a case report. The complexities to which we will return later. -

MSH, ACTH, and LHRH in Anorexia and Bulimia Nervosa Patients
Autoantibodies against ␣-MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients Sergueï O. Fetissov*†, Jarmila Hallman‡, Lars Oreland‡, Britt af Klinteberg§, Eva Grenba¨ ck¶, Anna-Lena Hulting¶, and Tomas Ho¨ kfelt* Departments of *Neuroscience and ¶Endocrinology, Karolinska Institute, SE-171 77 Stockholm, Sweden; ‡Department of Neuroscience, Biomedical Center, SE-751 24 Uppsala, Sweden; and §Department of Psychology, Stockholm University, SE-106 91 Stockholm, Sweden Contributed by Tomas Ho¨kfelt, October 30, 2002 The hypothalamic arcuate nucleus is involved in the control of Materials and Methods energy intake and expenditure and may participate in the patho- Human Sera. Sera from 57 female patients (ages 17–42) with genesis of eating disorders such as anorexia nervosa (AN) and eating disorders, diagnosed according to the Diagnostic and bulimia nervosa (BN). Two systems are of particular interest in this Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV; ref. 40), respect, synthesizing ␣-melanocyte-stimulating hormone (␣-MSH) were used in this study. Among them 28 AN patients (average and synthesizing neuropeptide Y, respectively. We report here that body weight Ϯ SD, 39.4 Ϯ 6.2 kg), 22 BN patients (66.1 Ϯ 25 kg), 42 of 57 (74%) AN and͞or BN patients studied had in their plasma and seven patients with combination of both AN and BN (47.1 Ϯ Abs that bind to melanotropes and͞or corticotropes in the rat 1.4 kg) were diagnosed. Sera from 13 healthy female volunteers pituitary. Among these sera, 8 were found to bind selectively to (age 20–41, 64.7 Ϯ 5.6 kg) served as control. -

Lack of Rapid Antidepressant Effects of Kir4.1 Channel Inhibitors in A
Pharmacology, Biochemistry and Behavior 176 (2019) 57–62 Contents lists available at ScienceDirect Pharmacology, Biochemistry and Behavior journal homepage: www.elsevier.com/locate/pharmbiochembeh Lack of rapid antidepressant effects of Kir4.1 channel inhibitors in a chronic social defeat stress model: Comparison with (R)-ketamine T Zhongwei Xionga,b, Kai Zhanga, Tamaki Ishimaa, Qian Rena, Min Maa, Yaoyu Pua, Lijia Changa, ⁎ Jincao Chenb, Kenji Hashimotoa, a Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba 260-8670, Japan b Department of Neurosurgery, Zhongnan Hospital of Wuhan University, Wuhan University, Wuhan 430071, PR China ARTICLE INFO ABSTRACT Keywords: A recent study demonstrated a key role of astroglial potassium channel Kir4.1 in the lateral habenula in de- Antidepressant pression. We investigated whether Kir4.1 protein is altered in the brain regions from susceptible mice after a Kir4.1 channel chronic social defeat stress (CSDS). Furthermore, we compared the rapid and sustained antidepressant actions of (R)-Ketamine Kir4.1 inhibitors (quinacrine and sertraline) and (R)-ketamine, (R)-enantiomer of rapid-acting antidepressant Social defeat stress model (R,S)-ketamine, in a CSDS model. Western blot analysis of Kir4.1 protein in the brain regions (prefrontal cortex, nucleus accumbens, hippocampus) from CSDS susceptible mice and control mice (no CSDS) was performed. Quinacrine (15, or 30 mg/kg), sertraline (20 mg/kg), (R)-ketamine (10 mg/kg), or vehicle was administered intraperitoneally to CSDS susceptible mice. Subsequently, locomotion test, tail suspension test (TST), forced swimming test (FST) and 1% sucrose preference test (SPT) were performed. There were no changes of Kir4.1 protein in the all regions between two groups. -

Diamandis Thesis
!"!#$ CHEMICAL GENETIC INTERROGATION OF NEURAL STEM CELLS: PHENOTYPE AND FUNCTION OF NEUROTRANSMITTER PATHWAYS IN NORMAL AND BRAIN TUMOUR INITIATING NEURAL PRECUSOR CELLS by Phedias Diamandis A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy. Department of Molecular Genetics University of Toronto © Copyright by Phedias Diamandis 2010 Phenotype and Function of Neurotransmitter Pathways in Normal and Brain Tumor Initiating Neural Precursor Cells Phedias Diamandis Doctor of Philosophy Department of Molecular Genetics University of Toronto 2010 &'(!)&*!% The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain brings promise for the treatment of neurological diseases and has yielded new insight into brain cancer. The complete repertoire of signaling pathways that governs these cells however remains largely uncharacterized. This thesis describes how chemical genetic approaches can be used to probe and better define the operational circuitry of the NSC. I describe the development of a small molecule chemical genetic screen of NSCs that uncovered an unappreciated precursor role of a number of neurotransmitter pathways commonly thought to operate primarily in the mature central nervous system (CNS). Given the similarities between stem cells and cancer, I then translated this knowledge to demonstrate that these neurotransmitter regulatory effects are also conserved within cultures of cancer stem cells. I then provide experimental and epidemiologically support for this hypothesis and suggest that neurotransmitter signals may also regulate the expansion of precursor cells that drive tumor growth in the brain. Specifically, I first evaluate the effects of neurochemicals in mouse models of brain tumors. I then outline a retrospective meta-analysis of brain tumor incidence rates in psychiatric patients presumed to be chronically taking neuromodulators similar to those identified in the initial screen. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau Et Al
US 20150202317A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0202317 A1 Rau et al. (43) Pub. Date: Jul. 23, 2015 (54) DIPEPTDE-BASED PRODRUG LINKERS Publication Classification FOR ALPHATIC AMNE-CONTAINING DRUGS (51) Int. Cl. A647/48 (2006.01) (71) Applicant: Ascendis Pharma A/S, Hellerup (DK) A638/26 (2006.01) A6M5/9 (2006.01) (72) Inventors: Harald Rau, Heidelberg (DE); Torben A 6LX3/553 (2006.01) Le?mann, Neustadt an der Weinstrasse (52) U.S. Cl. (DE) CPC ......... A61K 47/48338 (2013.01); A61 K3I/553 (2013.01); A61 K38/26 (2013.01); A61 K (21) Appl. No.: 14/674,928 47/48215 (2013.01); A61M 5/19 (2013.01) (22) Filed: Mar. 31, 2015 (57) ABSTRACT The present invention relates to a prodrug or a pharmaceuti Related U.S. Application Data cally acceptable salt thereof, comprising a drug linker conju (63) Continuation of application No. 13/574,092, filed on gate D-L, wherein D being a biologically active moiety con Oct. 15, 2012, filed as application No. PCT/EP2011/ taining an aliphatic amine group is conjugated to one or more 050821 on Jan. 21, 2011. polymeric carriers via dipeptide-containing linkers L. Such carrier-linked prodrugs achieve drug releases with therapeu (30) Foreign Application Priority Data tically useful half-lives. The invention also relates to pharma ceutical compositions comprising said prodrugs and their use Jan. 22, 2010 (EP) ................................ 10 151564.1 as medicaments. US 2015/0202317 A1 Jul. 23, 2015 DIPEPTDE-BASED PRODRUG LINKERS 0007 Alternatively, the drugs may be conjugated to a car FOR ALPHATIC AMNE-CONTAINING rier through permanent covalent bonds. -

Differential Mechanisms of Antianalgesia Induced by Endomorphin-1 and Endomorphin-2 in the Ventral Periaqueductal Gray of the Rat
0022-3565/05/3123-1257–1265$20.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 312, No. 3 Copyright © 2005 by The American Society for Pharmacology and Experimental Therapeutics 76224/1193072 JPET 312:1257–1265, 2005 Printed in U.S.A. Differential Mechanisms of Antianalgesia Induced by Endomorphin-1 and Endomorphin-2 in the Ventral Periaqueductal Gray of the Rat Maia Terashvili, Hsiang-en Wu, Randy J. Leitermann, Han-Sen Sun, Andrew D. Clithero, and Leon F. Tseng Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin Received August 13, 2004; accepted November 11, 2004 Downloaded from ABSTRACT The effects of pretreatment with endomorphin-1 (EM-1) and en- -opioid receptor antagonist 3-methoxynaltrexone (6.4 pmol) se- domorphin-2 (EM-2) given into the ventral periaqueductal gray lectively blocked EM-2- but not EM-1-induced antianalgesia. Pre- (vPAG) to induce antianalgesia against the tail-flick (TF) inhibition treatment with dynorphin A(1–17) antiserum reversed only EM-2- produced by morphine given into the vPAG were studied in rats. but not EM-1-induced antianalgesia. Pretreatment with antiserum jpet.aspetjournals.org Pretreatment with EM-1 (3.5–28 nmol) given into vPAG for 45 min against -endorphin, [Met5]enkephalin, [Leu5]enkephalin, sub- dose-dependently attenuated the TF inhibition produced by mor- stance P or cholecystokinin, or with ␦-opioid receptor antagonist phine (9 nmol) given into vPAG. Similarly, pretreatment with EM-2 naltrindole (2.2 nmol) or -opioid receptor antagonist norbinaltor- (1.7–7.0 nmol) for 45 min also attenuated the TF inhibition induced phimine (6.6 nmol) did not affect EM-2-induced antianalgesia. -

Five Decades of Research on Opioid Peptides: Current Knowledge and Unanswered Questions
Molecular Pharmacology Fast Forward. Published on June 2, 2020 as DOI: 10.1124/mol.120.119388 This article has not been copyedited and formatted. The final version may differ from this version. File name: Opioid peptides v45 Date: 5/28/20 Review for Mol Pharm Special Issue celebrating 50 years of INRC Five decades of research on opioid peptides: Current knowledge and unanswered questions Lloyd D. Fricker1, Elyssa B. Margolis2, Ivone Gomes3, Lakshmi A. Devi3 1Department of Molecular Pharmacology, Albert Einstein College of Medicine, Bronx, NY 10461, USA; E-mail: [email protected] 2Department of Neurology, UCSF Weill Institute for Neurosciences, 675 Nelson Rising Lane, San Francisco, CA 94143, USA; E-mail: [email protected] 3Department of Pharmacological Sciences, Icahn School of Medicine at Mount Sinai, Annenberg Downloaded from Building, One Gustave L. Levy Place, New York, NY 10029, USA; E-mail: [email protected] Running Title: Opioid peptides molpharm.aspetjournals.org Contact info for corresponding author(s): Lloyd Fricker, Ph.D. Department of Molecular Pharmacology Albert Einstein College of Medicine 1300 Morris Park Ave Bronx, NY 10461 Office: 718-430-4225 FAX: 718-430-8922 at ASPET Journals on October 1, 2021 Email: [email protected] Footnotes: The writing of the manuscript was funded in part by NIH grants DA008863 and NS026880 (to LAD) and AA026609 (to EBM). List of nonstandard abbreviations: ACTH Adrenocorticotrophic hormone AgRP Agouti-related peptide (AgRP) α-MSH Alpha-melanocyte stimulating hormone CART Cocaine- and amphetamine-regulated transcript CLIP Corticotropin-like intermediate lobe peptide DAMGO D-Ala2, N-MePhe4, Gly-ol]-enkephalin DOR Delta opioid receptor DPDPE [D-Pen2,D- Pen5]-enkephalin KOR Kappa opioid receptor MOR Mu opioid receptor PDYN Prodynorphin PENK Proenkephalin PET Positron-emission tomography PNOC Pronociceptin POMC Proopiomelanocortin 1 Molecular Pharmacology Fast Forward.