Adrenocorticotropic Hormone in Human Pituitary and Pituitary Adenomas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Kisspeptin Neurons in Reproduction and Metabolism
238 3 Journal of C J L Harter, G S Kavanagh Kisspeptin, reproduction and 238:3 R173–R183 Endocrinology et al. metabolism REVIEW The role of kisspeptin neurons in reproduction and metabolism Campbell J L Harter*, Georgia S Kavanagh* and Jeremy T Smith School of Human Sciences, The University of Western Australia, Perth, Western Australia, Australia Correspondence should be addressed to J T Smith: [email protected] *(C J L Harter and G S Kavanagh contributed equally to this work) Abstract Kisspeptin is a neuropeptide with a critical role in the function of the hypothalamic– Key Words pituitary–gonadal (HPG) axis. Kisspeptin is produced by two major populations of f Kiss1 neurons located in the hypothalamus, the rostral periventricular region of the third f hypothalamus ventricle (RP3V) and arcuate nucleus (ARC). These neurons project to and activate f fertility gonadotrophin-releasing hormone (GnRH) neurons (acting via the kisspeptin receptor, f energy homeostasis Kiss1r) in the hypothalamus and stimulate the secretion of GnRH. Gonadal sex steroids f glucose metabolism stimulate kisspeptin neurons in the RP3V, but inhibit kisspeptin neurons in the ARC, which is the underlying mechanism for positive- and negative feedback respectively, and it is now commonly accepted that the ARC kisspeptin neurons act as the GnRH pulse generator. Due to kisspeptin’s profound effect on the HPG axis, a focus of recent research has been on afferent inputs to kisspeptin neurons and one specific area of interest has been energy balance, which is thought to facilitate effects such as suppressing fertility in those with under- or severe over-nutrition. -

Serum Levels of Spexin and Kisspeptin Negatively Correlate with Obesity and Insulin Resistance in Women
Physiol. Res. 67: 45-56, 2018 https://doi.org/10.33549/physiolres.933467 Serum Levels of Spexin and Kisspeptin Negatively Correlate With Obesity and Insulin Resistance in Women P. A. KOŁODZIEJSKI1, E. PRUSZYŃSKA-OSZMAŁEK1, E. KOREK4, M. SASSEK1, D. SZCZEPANKIEWICZ1, P. KACZMAREK1, L. NOGOWSKI1, P. MAĆKOWIAK1, K. W. NOWAK1, H. KRAUSS4, M. Z. STROWSKI2,3 1Department of Animal Physiology and Biochemistry, Poznan University of Life Sciences, Poznan, Poland, 2Department of Hepatology and Gastroenterology & The Interdisciplinary Centre of Metabolism: Endocrinology, Diabetes and Metabolism, Charité-University Medicine Berlin, Berlin, Germany, 3Department of Internal Medicine, Park-Klinik Weissensee, Berlin, Germany, 4Department of Physiology, Karol Marcinkowski University of Medical Science, Poznan, Poland Received August 18, 2016 Accepted June 19, 2017 On-line November 10, 2017 Summary Corresponding author Spexin (SPX) and kisspeptin (KISS) are novel peptides relevant in P. A. Kolodziejski, Department of Animal Physiology and the context of regulation of metabolism, food intake, puberty and Biochemistry, Poznan University of Life Sciences, Wolynska Street reproduction. Here, we studied changes of serum SPX and KISS 28, 60-637 Poznan, Poland. E-mail: [email protected] levels in female non-obese volunteers (BMI<25 kg/m2) and obese patients (BMI>35 kg/m2). Correlations between SPX or Introduction KISS with BMI, McAuley index, QUICKI, HOMA IR, serum levels of insulin, glucagon, leptin, adiponectin, orexin-A, obestatin, Kisspeptin (KISS) and spexin (SPX) are peptides ghrelin and GLP-1 were assessed. Obese patients had lower SPX involved in regulation of body weight, metabolism and and KISS levels as compared to non-obese volunteers (SPX: sexual functions. In 2014, Kim and coworkers showed that 4.48±0.19 ng/ml vs. -

Phenotype Microarrays Panels PM-M1 to PM-M14
Phenotype MicroArrays™ Panels PM-M1 to PM-M14 for Phenotypic Characterization of Mammalian Cells Assays: Energy Metabolism Pathways Ion and Hormone Effects on Cells Sensitivity to Anti-Cancer Agents and for Optimizing Culture Conditions for Mammalian Cells PRODUCT DESCRIPTIONS AND INSTRUCTIONS FOR USE PM-M1 Cat. #13101 PM-M2 Cat. #13102 PM-M3 Cat. #13103 PM-M4 Cat. #13104 PM-M5 Cat. #13105 PM-M6 Cat. #13106 PM-M7 Cat. #13107 PM-M8 Cat. #13108 PM-M11 Cat. #13111 PM-M12 Cat. #13112 PM-M13 Cat. #13113 PM-M14 Cat. #13114 © 2016 Biolog, Inc. All rights reserved Printed in the United States of America 00P 134 Rev F February 2020 - 1 - CONTENTS I. Introduction ...................................................................................................... 2 a. Overview ................................................................................................... 2 b. Background ............................................................................................... 2 c. Uses ........................................................................................................... 2 d. Advantages ................................................................................................ 3 II. Product Description, PM-M1 to M4 ................................................................ 3 III. Protocols, PM-M1 to M4 ................................................................................. 7 a. Materials Required .................................................................................... 7 b. Determination -

Purification of a Galanin Receptor from Pig Brain
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 3845-3849, May 1993 Neurobiology Purification of a galanin receptor from pig brain YAOHUI CHEN*, ALAIN FOURNIERt, ALAIN COUVINEAU*, MARC LABURTHE*, AND BRIGITTE AMIRANOFF*t *Laboratoire de Biologie and Physiologie des Cellules Digestives, Institut National de la Sant6 et de la Recherche Mddicale, U 239, 16 Rue Henri Huchard-75018 Paris, France; and tUniversitd du Quebec, Institut National de la Recherche Scientifique, INRS-Santd, 245 Boulevard Hymus, Pointe Claire, Qudbec, H9R1G6, Canada Communicated by Tomas Hokfelt, January 4, 1993 ABSTRACT A galanin receptor protein was solubilized lished data), we report the purification of a galanin receptor with 3-[(3-cholamidopropyl)dimethylammonio]-1-propane- from pig brain, a rich source of receptors that is available in sulfonate (CHAPS) from pig brain membranes and then pu- large amounts. This represents a basic step toward knowl- rified by single-step affinity chromatography. The product edge of the pharmacology and biochemistry of galanin re- exhibits saturable and specific binding for galanin with a ceptors and should lead to a better understanding of their binding activity of 17 nmol/mg of protein and a dissociation expression in the organism. constant (Kd) of 10 nM. This represents a 300,000-fold puri- fication over the detergent-solubilized fraction with a final recovery of 31% of the initial membrane galanin binding METHODS activity. Gel electrophoresis of the affinity-purified material Materials. Synthetic porcine galanin, glucagon, vasoactive showed a single polypeptide of 54 kDa by silver staining and intestinal peptide, synthetic neurotensin, substance P, baci- after radioiodination. Cross-linking of a purified fraction af- tracin, leupeptin, pepstatin A, GTP, GDP, guanosine 5'-[13,v- rmity-labeled with 125I-labeled galanin revealed a single band imido]triphosphate, cholesteryl hemisuccinate, 3-[(3-cholami- for the galanin-receptor complex at 57 kDa. -

Reorganization of Neural Peptidergic Eminence After Hypophysectomy
The Journal of Neuroscience, October 1994, 14(10): 59966012 Reorganization of Neural Peptidergic Systems Median Eminence after Hypophysectomy Marcel0 J. Villar, Bjiirn Meister, and Tomas Hiikfelt Department of Neuroscience, The Berzelius Laboratory, Karolinska Institutet, Stockholm, 171 77 Sweden Earlier studies have shown the formation of a novel neural crease to a final stage of a few, strongly immunoreactive lobe after hypophysectomy, an experimental manipulation fibers in the external layer at longer survival times. Vaso- that causes transection of neurohypophyseal nerve fibers active intestinal polypeptide (VIP)- and peptide histidine- and removal of pituitary hormones. The mechanisms that isoleucine (PHI)-IR fibers in hypophysectomized animals had underly this regenerative process are poorly understood. already contacted portal vessels 5 d after hypophysectomy, The localization and number of peptide-immunoreactive and from then on progressively increased in numbers. Fi- (-IR) fibers in the median eminence were studied in normal nally, most of the peptide fibers described above formed rats and in rats at different times of survival after hypophy- dense innervation patterns around the large blood vessels sectomy using indirect immunofluorescence histochemistry. along the lateral borders of the median eminence. The number of vasopressin (VP)-IR fibers increased in the The present results show that hypophysectomy induces external layer of the median eminence in 5 d hypophysec- a wide variety of changes in hypothalamic neurosecretory tomized rats. Oxytocin (OXY)-IR fibers decreased in the in- fibers. Not only is the expression of several peptides in these ternal layer and progressively extended into the external fibers modified following different survival times, but a re- layer. -
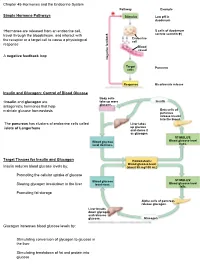
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -

Male-Predominant Galanin Mediates Androgen-Dependent Aggressive
RESEARCH ARTICLE Male-predominant galanin mediates androgen-dependent aggressive chases in medaka Junpei Yamashita1, Akio Takeuchi1, Kohei Hosono1†, Thomas Fleming1, Yoshitaka Nagahama2, Kataaki Okubo1* 1Department of Aquatic Bioscience, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan; 2Division of Reproductive Biology, National Institute for Basic Biology, Okazaki, Japan Abstract Recent studies in mice demonstrate that a subset of neurons in the medial preoptic area (MPOA) that express galanin play crucial roles in regulating parental behavior in both sexes. However, little information is available on the function of galanin in social behaviors in other species. Here, we report that, in medaka, a subset of MPOA galanin neurons occurred nearly exclusively in males, resulting from testicular androgen stimulation. Galanin-deficient medaka showed a greatly reduced incidence of male–male aggressive chases. Furthermore, while treatment of female medaka with androgen induced male-typical aggressive acts, galanin deficiency in these females attenuated the effect of androgen on chases. Given their male-biased and androgen- dependent nature, the subset of MPOA galanin neurons most likely mediate androgen-dependent male–male chases. Histological studies further suggested that variability in the projection targets of the MPOA galanin neurons may account for the species-dependent functional differences in these evolutionarily conserved neural substrates. *For correspondence: [email protected] Present address: †School of Life Science and Technology, Tokyo Introduction Institute of Technology, Yokohama, Japan Almost all animals interact socially with conspecifics at some stage of their lives (e.g. for territorial/ resource disputes, mating, and parenting) (Hofmann et al., 2014; Chen and Hong, 2018). -
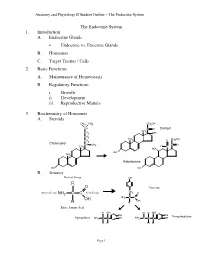
Lecture Outline
Anatomy and Physiology II Student Outline – The Endocrine System The Endocrine System 1. Introduction A. Endocrine Glands • Endocrine vs. Exocrine Glands B. Hormones C. Target Tissues / Cells 2. Basic Functions A. Maintenance of Homeostasis B. Regulatory Functions i. Growth ii. Development iii. Reproductive Matters 3. Biochemistry of Hormones A. Steroids CH3 CH3 CH2OH CH C O Cortisol CH CH2 3 OH CH2 CH CH OH CH 3 O 2 2 Cholesterol CH CH C O 3 H CH3 C HO HO CH 3 Aldosterone HO HO B. Amines Radical Group OH R O Tyrosine CH Amino Group NH2 C C Acid Group 2 O NH2 C C OH OH H H Basic Amino Acid H OH H H OH OH OH Norepinephrine Epinephrine NH2 C C OH NH2 C C C OH H H H H Page 1 Anatomy and Physiology II Student Outline – The Endocrine System C. Peptides • Antidiuretic Hormone • Oxytocin Oxytocin Antidiuretic Hormone Tyr Tyr Cys Cys Ileu Phe Glu Glu Cys Pro Leu Gly Cys Pro Arg Gly Asp Asp D. Proteins E. Glycoproteins 4. Feedback Control System A. Negative Feedback System (See Endocrine Pathways Handout: “Control Paradigm (Negative Feedback System)) i. Example: (See Endocrine Pathways Handout: “Negative Feedback Example”) B. Positive Feedback System (See Endocrine Pathways Handout: “Positive Feedback Example”) i. Child Birth and Oxytocin Page 2 Anatomy and Physiology II Student Outline – The Endocrine System 5. Mechanisms of Hormone Control A. Fixed-Membrane-Receptor Mechanism ii. Mechanism Inactive ATP Enzyme 1 Inactive cAMP Active Enzyme 2 Enzyme 1 (Secondary Active Messenger) Enzyme 2 Inactive Inactive Enzyme 4 ActiveEnzyme 3 Enzyme 3 Altered Active Cell Function Enzyme 4 ii. -

Ectopic Hormone Production by Malignant Tumors
ANNALS O F CLINICAL AND LABORATORY SCIENCE, Vol. 9, No. 4 Copyright © 1979, Institute for Clinical Science, Inc. Ectopic Hormone Production by Malignant Tumors IRWIN J. HOLLANDER, M.D. and GONZALO E. APONTE, M.D. Department of Pathology, Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA 19107 ABSTRACT Malignant tumors of nonendocrine tissues may produce ectopic hor mones. The most likely mechanism is depression of genes which code for hormones. Ectopic hormones are invariably peptides, and each is identical to some peptide product of an endocrine gland. However, the majority of ectopic hormones occur as biologically inactive precursors or subunits and therefore remain occult unless they are specifically sought. When appropri ate assays are made for such inactive forms, it is found that ectopic produc tion of hormone-like peptides occurs frequently. Clinical syndromes result only in the relatively rare patients in whom a biologically active form is synthesized in large quantities. Laboratory research in this area improves our understanding of genetic control mechanisms in neoplasia. Ectopic hormones may be of limited use in diagnosis of cancer, especially when multiple markers are measured simultaneously. Introduction Ectopic hormone production is synthe sis of a hormone by tissues which do not To most of us, the ectopic synthesis of normally produce that hormone. This def hormones by malignant tumors brings to inition implies, of course, that all of the mind a rare patient whose vigorous normal sites of origin of the hormone are workup by an enthusiastic endocrin known, but this assumption conceals ologist merited a case report. The complexities to which we will return later. -

MSH, ACTH, and LHRH in Anorexia and Bulimia Nervosa Patients
Autoantibodies against ␣-MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients Sergueï O. Fetissov*†, Jarmila Hallman‡, Lars Oreland‡, Britt af Klinteberg§, Eva Grenba¨ ck¶, Anna-Lena Hulting¶, and Tomas Ho¨ kfelt* Departments of *Neuroscience and ¶Endocrinology, Karolinska Institute, SE-171 77 Stockholm, Sweden; ‡Department of Neuroscience, Biomedical Center, SE-751 24 Uppsala, Sweden; and §Department of Psychology, Stockholm University, SE-106 91 Stockholm, Sweden Contributed by Tomas Ho¨kfelt, October 30, 2002 The hypothalamic arcuate nucleus is involved in the control of Materials and Methods energy intake and expenditure and may participate in the patho- Human Sera. Sera from 57 female patients (ages 17–42) with genesis of eating disorders such as anorexia nervosa (AN) and eating disorders, diagnosed according to the Diagnostic and bulimia nervosa (BN). Two systems are of particular interest in this Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV; ref. 40), respect, synthesizing ␣-melanocyte-stimulating hormone (␣-MSH) were used in this study. Among them 28 AN patients (average and synthesizing neuropeptide Y, respectively. We report here that body weight Ϯ SD, 39.4 Ϯ 6.2 kg), 22 BN patients (66.1 Ϯ 25 kg), 42 of 57 (74%) AN and͞or BN patients studied had in their plasma and seven patients with combination of both AN and BN (47.1 Ϯ Abs that bind to melanotropes and͞or corticotropes in the rat 1.4 kg) were diagnosed. Sera from 13 healthy female volunteers pituitary. Among these sera, 8 were found to bind selectively to (age 20–41, 64.7 Ϯ 5.6 kg) served as control. -

Localization and Role of Galanin in the Thyroid Gland of Podarcis Sicula Lizard
JOURNAL OF EXPERIMENTAL ZOOLOGY 311A:199–206 (2009) A Journal of Integrative Biology Localization and Role of Galanin in the Thyroid Gland of Podarcis sicula Lizard (Reptilia, Lacertide) ROSARIA SCIARRILLO1Ã, ANNA CAPALDO2, SALVATORE VALIANTE2, 2 2 VINCENZA LAFORGIA , AND MARIA DE FALCO 1Department of Biological and Environmental Sciences, University of Sannio, Benevento, Italy 2Department of Evolutive and Comparative Biology, University of Naples ‘‘Federico II,’’ Naples, Italy ABSTRACT Galanin (GAL) is a 29-amino acid residue neuropeptide, which was initially isolated from porcine intestine extracts and since then, widely found in a variety of vertebrate organs, in correlation with multiple neuro-hormonal actions exerted and so receiving a constantly growing attention. Moreover, although the studies undertaken so far suggest a local intrathyroidal peptidergic regulatory action, the exact role of GAL on thyroid gland remains to be established. The aim of this study was to determine in the lizard, Podarcis sicula, (1) the presence of GAL immunoreactivity in the thyroid gland and (2) the short- and long-term effects of in vivo GAL administration by intraperitoneal injection on thyroid gland physiology. First of all, the presence of GAL in the thyroid gland of P. sicula was demonstrated by immunohistochemical technique (avidin–biotin–peroxidase complex—ABC method). Second, the role of GAL in the control of thyroid gland activity was studied in vivo using light microscopy (LM) technique coupled to a specific radioimmunoassay for thyroid-stimulating hormone (TSH) and thyroid hormones (T4 and T3). Prolonged GAL administration [(0.4 mg/100 g body wt)/day] increased T4 and T3 release, but decreased the plasma concentration of TSH. -

Developmental Expression Oe Neurotensin and Galanin In
Biomedical Research 16 (5) 281-286, 1995 i 1| i 2 DEVELOPMENTAL EXPRESSION OE NEUROTENSIN AND GALANIN § IN THE RAT GASTROINTESTINAL TRACT 2 i i MUNEO OKA‘, NIMA KHANDAN-—NIA2, PHILIP JoNEs2, MOHAMMAD GHATEI2 and STEPHEN ROBERT BLOOM2 ‘Department of Endocrinology, Internal Medicine, Dokkyo University School of Medicine, Mibu, Tochigi 321-02, Japan, and 2Department of Medicine, Royal Postgraduate Medical School, Hammersmith Hospital, Du Cane Road, London W12 ONN, U.K. ABSTRACT To characterize and compare the developmental patterns of neurotensin (NT) and galanin (GAL) expression in the gastrointestinal tract, we measured both peptide content and mRNA concentrations in different regions of the rat gastrointestinal tract at different times during fetal and postnatal development. The abundance of NT mRNA in the jejunum and ileum increased from birth and peaked on day 7, subsequently decreased by day 21. In con- trast, expression of NT mRNA in the stomach and colon remained very low. Changes in NT peptide content parallelled that in NT mRNA. On the other hand, the abundance of GAL mRNA, which was expressed in all regions of the gastrointestinal tract, gradually increased until day 21 following birth. GAL peptide content increased up until day 7; thereafter, the levels of this peptide remained relatively unchanged. Thus, the gene expres- sion of NT and GAL each demonstrates a specific pattern of tissue distribution and a dif- ferent developmental pattern. These data suggest that NT and GAL gene expression and peptide content are developmentally regulated and that NT and GAL each play a different role in the gastrointestinal tract during development. Previous studies have suggested important roles for distribution of these neuropeptides during gut several neuropeptides in maintaining the functional development should provide a better understanding and structural integrity of the gut.