Mechanisms That Prevent Recovery in Prolonged ICU Patients Also Underlie Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phenotype Microarrays Panels PM-M1 to PM-M14
Phenotype MicroArrays™ Panels PM-M1 to PM-M14 for Phenotypic Characterization of Mammalian Cells Assays: Energy Metabolism Pathways Ion and Hormone Effects on Cells Sensitivity to Anti-Cancer Agents and for Optimizing Culture Conditions for Mammalian Cells PRODUCT DESCRIPTIONS AND INSTRUCTIONS FOR USE PM-M1 Cat. #13101 PM-M2 Cat. #13102 PM-M3 Cat. #13103 PM-M4 Cat. #13104 PM-M5 Cat. #13105 PM-M6 Cat. #13106 PM-M7 Cat. #13107 PM-M8 Cat. #13108 PM-M11 Cat. #13111 PM-M12 Cat. #13112 PM-M13 Cat. #13113 PM-M14 Cat. #13114 © 2016 Biolog, Inc. All rights reserved Printed in the United States of America 00P 134 Rev F February 2020 - 1 - CONTENTS I. Introduction ...................................................................................................... 2 a. Overview ................................................................................................... 2 b. Background ............................................................................................... 2 c. Uses ........................................................................................................... 2 d. Advantages ................................................................................................ 3 II. Product Description, PM-M1 to M4 ................................................................ 3 III. Protocols, PM-M1 to M4 ................................................................................. 7 a. Materials Required .................................................................................... 7 b. Determination -
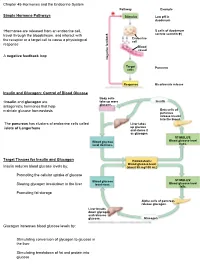
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -

Feedback Regulation of Growth Hormone Synthesis and Secretion in Fish and the Emerging Concept of Intrapituitary Feedback Loop ☆ ⁎ Anderson O.L
http://www.paper.edu.cn Comparative Biochemistry and Physiology, Part A 144 (2006) 284–305 Review Feedback regulation of growth hormone synthesis and secretion in fish and the emerging concept of intrapituitary feedback loop ☆ ⁎ Anderson O.L. Wong , Hong Zhou, Yonghua Jiang, Wendy K.W. Ko Department of Zoology, University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China Received 29 July 2005; received in revised form 21 November 2005; accepted 21 November 2005 Available online 9 January 2006 Abstract Growth hormone (GH) is known to play a key role in the regulation of body growth and metabolism. Similar to mammals, GH secretion in fish is under the control of hypothalamic factors. Besides, signals generated within the pituitary and/or from peripheral tissues/organs can also exert a feedback control on GH release by effects acting on both the hypothalamus and/or anterior pituitary. Among these feedback signals, the functional role of IGF is well conserved from fish to mammals. In contrast, the effects of steroids and thyroid hormones are more variable and appear to be species-specific. Recently, a novel intrapituitary feedback loop regulating GH release and GH gene expression has been identified in fish. This feedback loop has three functional components: (i) LH induction of GH release from somatotrophs, (ii) amplification of GH secretion by GH autoregulation in somatotrophs, and (iii) GH feedback inhibition of LH release from neighboring gonadotrophs. In this article, the mechanisms for feedback control of GH synthesis and secretion are reviewed and functional implications of this local feedback loop are discussed. This intrapituitary feedback loop may represent a new facet of pituitary research with potential applications in aquaculture and clinical studies. -
Lecture Outline
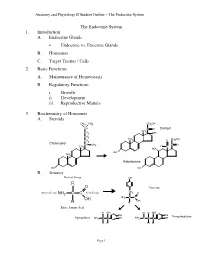
Anatomy and Physiology II Student Outline – The Endocrine System The Endocrine System 1. Introduction A. Endocrine Glands • Endocrine vs. Exocrine Glands B. Hormones C. Target Tissues / Cells 2. Basic Functions A. Maintenance of Homeostasis B. Regulatory Functions i. Growth ii. Development iii. Reproductive Matters 3. Biochemistry of Hormones A. Steroids CH3 CH3 CH2OH CH C O Cortisol CH CH2 3 OH CH2 CH CH OH CH 3 O 2 2 Cholesterol CH CH C O 3 H CH3 C HO HO CH 3 Aldosterone HO HO B. Amines Radical Group OH R O Tyrosine CH Amino Group NH2 C C Acid Group 2 O NH2 C C OH OH H H Basic Amino Acid H OH H H OH OH OH Norepinephrine Epinephrine NH2 C C OH NH2 C C C OH H H H H Page 1 Anatomy and Physiology II Student Outline – The Endocrine System C. Peptides • Antidiuretic Hormone • Oxytocin Oxytocin Antidiuretic Hormone Tyr Tyr Cys Cys Ileu Phe Glu Glu Cys Pro Leu Gly Cys Pro Arg Gly Asp Asp D. Proteins E. Glycoproteins 4. Feedback Control System A. Negative Feedback System (See Endocrine Pathways Handout: “Control Paradigm (Negative Feedback System)) i. Example: (See Endocrine Pathways Handout: “Negative Feedback Example”) B. Positive Feedback System (See Endocrine Pathways Handout: “Positive Feedback Example”) i. Child Birth and Oxytocin Page 2 Anatomy and Physiology II Student Outline – The Endocrine System 5. Mechanisms of Hormone Control A. Fixed-Membrane-Receptor Mechanism ii. Mechanism Inactive ATP Enzyme 1 Inactive cAMP Active Enzyme 2 Enzyme 1 (Secondary Active Messenger) Enzyme 2 Inactive Inactive Enzyme 4 ActiveEnzyme 3 Enzyme 3 Altered Active Cell Function Enzyme 4 ii. -

Ectopic Hormone Production by Malignant Tumors
ANNALS O F CLINICAL AND LABORATORY SCIENCE, Vol. 9, No. 4 Copyright © 1979, Institute for Clinical Science, Inc. Ectopic Hormone Production by Malignant Tumors IRWIN J. HOLLANDER, M.D. and GONZALO E. APONTE, M.D. Department of Pathology, Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA 19107 ABSTRACT Malignant tumors of nonendocrine tissues may produce ectopic hor mones. The most likely mechanism is depression of genes which code for hormones. Ectopic hormones are invariably peptides, and each is identical to some peptide product of an endocrine gland. However, the majority of ectopic hormones occur as biologically inactive precursors or subunits and therefore remain occult unless they are specifically sought. When appropri ate assays are made for such inactive forms, it is found that ectopic produc tion of hormone-like peptides occurs frequently. Clinical syndromes result only in the relatively rare patients in whom a biologically active form is synthesized in large quantities. Laboratory research in this area improves our understanding of genetic control mechanisms in neoplasia. Ectopic hormones may be of limited use in diagnosis of cancer, especially when multiple markers are measured simultaneously. Introduction Ectopic hormone production is synthe sis of a hormone by tissues which do not To most of us, the ectopic synthesis of normally produce that hormone. This def hormones by malignant tumors brings to inition implies, of course, that all of the mind a rare patient whose vigorous normal sites of origin of the hormone are workup by an enthusiastic endocrin known, but this assumption conceals ologist merited a case report. The complexities to which we will return later. -

MSH, ACTH, and LHRH in Anorexia and Bulimia Nervosa Patients
Autoantibodies against ␣-MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients Sergueï O. Fetissov*†, Jarmila Hallman‡, Lars Oreland‡, Britt af Klinteberg§, Eva Grenba¨ ck¶, Anna-Lena Hulting¶, and Tomas Ho¨ kfelt* Departments of *Neuroscience and ¶Endocrinology, Karolinska Institute, SE-171 77 Stockholm, Sweden; ‡Department of Neuroscience, Biomedical Center, SE-751 24 Uppsala, Sweden; and §Department of Psychology, Stockholm University, SE-106 91 Stockholm, Sweden Contributed by Tomas Ho¨kfelt, October 30, 2002 The hypothalamic arcuate nucleus is involved in the control of Materials and Methods energy intake and expenditure and may participate in the patho- Human Sera. Sera from 57 female patients (ages 17–42) with genesis of eating disorders such as anorexia nervosa (AN) and eating disorders, diagnosed according to the Diagnostic and bulimia nervosa (BN). Two systems are of particular interest in this Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV; ref. 40), respect, synthesizing ␣-melanocyte-stimulating hormone (␣-MSH) were used in this study. Among them 28 AN patients (average and synthesizing neuropeptide Y, respectively. We report here that body weight Ϯ SD, 39.4 Ϯ 6.2 kg), 22 BN patients (66.1 Ϯ 25 kg), 42 of 57 (74%) AN and͞or BN patients studied had in their plasma and seven patients with combination of both AN and BN (47.1 Ϯ Abs that bind to melanotropes and͞or corticotropes in the rat 1.4 kg) were diagnosed. Sera from 13 healthy female volunteers pituitary. Among these sera, 8 were found to bind selectively to (age 20–41, 64.7 Ϯ 5.6 kg) served as control. -

Alternative Processing of Bovine Growth Hormone Mrna
Proc. Natl. Acad. Sci. USA Vol. 84, pp. 2673-2677, May 1987 Biochemistry Alternative processing of bovine growth hormone mRNA: Nonsplicing of the final intron predicts a high molecular weight variant of bovine growth hormone (intron D/alternative reading frame/growth hormone-related polypeptide) ROBERT K. HAMPSON AND FRITZ M. ROTTMAN Department of Molecular Biology and Microbiology, Case Western Reserve University School of Medicine, 2119 Abington Road, Cleveland, OH 44106 Communicated by Lester 0. Krampitz, January 2, 1987 (received for review September 10, 1986) ABSTRACT We have detected a variant species of bovine MATERIALS AND METHODS growth hormone mRNA in bovine pituitary tissue and in a stably transfected bovine growth hormone-producing cell line. CHO 14-10-4 Cell Line. The Chinese hamster ovary (CHO) Analysis of this variant mRNA indicated that the last inter- cell line, CHO 14-10-4, utilized in these studies was gener- vening sequence (intron D) had not been removed by splicing. ously provided by Leonard Post. These cells were derived Inspection of the sequence of intron D reveals an open reading frame through the entire intron, with a termination codon from the DBX-11 cell line of dihydrofolate reductase-nega- encountered 50 nucleotides into the fifth exon, which is shifted tive CHO cells (6) and have been stably transfected with an from the normal reading frame in this variant mRNA. If expression plasmid containing the bovine growth hormone translated, this variant mRNA would encode a growth hor- gene. This expression plasmid, pSV2Cdhfr (Fig. 1), contains mone-related polypeptide having 125 amino-terminal amino the BamHI/EcoRI fragment of the bovine growth hormone acids identical to wild-type growth hormone, followed by 108 genomic clone (2) in the plasmid pSV2dhfr (7) situated carboxyl-terminal amino acids encoded by the 274 bases of downstream from a 760-base-pair Sau3A fragment containing intron D along with the first 50 nucleotides of exon 5. -

Download?Doi=10.1.1.640.8406&Rep=Rep1&T Ype=Pdf
UC Riverside UC Riverside Electronic Theses and Dissertations Title The Effects of Temperature, Salinity, and Bifenthrin on the Behavior and Neuroendocrinology of Juvenile Salmon and Trout Permalink https://escholarship.org/uc/item/9jv04705 Author Giroux, Marissa Sarah Publication Date 2019 License https://creativecommons.org/licenses/by-nd/4.0/ 4.0 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA RIVERSIDE The Effects of Temperature, Salinity, and Bifenthrin on the Behavior and Neuroendocrinology of Juvenile Salmon and Trout A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Environmental Toxicology by Marissa S. Giroux June 2019 Dissertation Committee: Dr. Daniel Schlenk, Chairperson Dr. David Volz Dr. Andrew Gray Copyright by Marissa S. Giroux 2019 The Dissertation of Marissa S. Giroux is approved: Committee Chairperson University of California, Riverside ACKNOWLEDGEMENTS Without the support, guidance, training, and time of many people this dissertation would not be possible. First, I would like to thank my Advisor, Dr. Daniel Schlenk for his help in in applying for fellowships, guidance in experimental design, taking the time to help me network, and giving me the opportunity to teach and mentor in both the lab and classroom. I would also like to thank my committee members, Dr. David Volz and Dr. Andrew Gray, for their wonderful recommendations and advice throughout my years at UC-Riverside. I have immense gratitude for my lab family, both past and present, who trained me, supported me, and taught me all of the “other” things you learn in grad school. -

Growth Hormone Therapy in Children with Chronic Renal Failure
Eurasian J Med 2015; 47: 62-5 Review Growth Hormone Therapy in Children with Chronic Renal Failure Kronik Böbrek Yetmezliği olan Çocuklarda Büyüme Hormonu Tedavisi Atilla Cayir1, Celalettin Kosan2 1Department of Pediatric Endocrinology, Regional Training and Research Hospital, Erzurum, Turkey 2Department of Pediatric Nephrology, Ataturk University Faculty of Medicine, Erzurum, Turkey Abstract Özet Growth is impaired in a chronic renal failure. Anemia, acidosis, re- Kronik böbrek yetersizliğinde büyüme bozulmaktadır. Anemi, asidoz, duced intake of calories and protein, decreased synthesis of vitamin kalori ve protein alımının azalması, azalmış vitamin D sentezi ve artmış D and increased parathyroid hormone levels, hyperphosphatemia, parathormon düzeyi, hiperfosfatemi, renal osteodistrofi ve büyüme renal osteodystrophy and changes in growth hormone-insulin-like hormonu, insülin benzeri growth faktör ile gonadotropin- gonadal growth factor and the gonadotropin-gonadal axis are implicated in akstaki değişiklikler büyümenin yetersiz olmasından sorumlu tutul- this study. Growth is adversely affected by immunosuppressives and maktadır. Böbrek transplantasyonundan sonra ise immunosupressifler corticosteroids after kidney transplantation. Treating metabolic dis- ve kortikosteroidlerin etkileri ile büyüme olumsuz olarak etkilenmek- orders using the recombinant human growth hormone is an effective tedir. Metabolik bozuklukların düzeltilmesine rağmen büyüme hızı option for patients with inadequate growth rates. yetersiz olan olgularda rekombinant insan büyüme hormonu iyi bir Keywords: Child, chronic renal failure, growth hormone, therapy tedavi seçeneğidir. Anahtar Kelimeler: Çocuk, kronik böbrek yetersizliği, büyüme hor- monu, tedavi Introduction mone takes place with glomerular filtration and breakdown in the proximal tubules. In CRF, a decrease in the rate of glo- Inadequate growth is a widespread problem in children merular filtration leads to impairment of the metabolic clear- with chronic renal failure (CRF). -

A Bioinformatics Model of Human Diseases on the Basis Of
SUPPLEMENTARY MATERIALS A Bioinformatics Model of Human Diseases on the basis of Differentially Expressed Genes (of Domestic versus Wild Animals) That Are Orthologs of Human Genes Associated with Reproductive-Potential Changes Vasiliev1,2 G, Chadaeva2 I, Rasskazov2 D, Ponomarenko2 P, Sharypova2 E, Drachkova2 I, Bogomolov2 A, Savinkova2 L, Ponomarenko2,* M, Kolchanov2 N, Osadchuk2 A, Oshchepkov2 D, Osadchuk2 L 1 Novosibirsk State University, Novosibirsk 630090, Russia; 2 Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences, Novosibirsk 630090, Russia; * Correspondence: [email protected]. Tel.: +7 (383) 363-4963 ext. 1311 (M.P.) Supplementary data on effects of the human gene underexpression or overexpression under this study on the reproductive potential Table S1. Effects of underexpression or overexpression of the human genes under this study on the reproductive potential according to our estimates [1-5]. ↓ ↑ Human Deficit ( ) Excess ( ) # Gene NSNP Effect on reproductive potential [Reference] ♂♀ NSNP Effect on reproductive potential [Reference] ♂♀ 1 increased risks of preeclampsia as one of the most challenging 1 ACKR1 ← increased risk of atherosclerosis and other coronary artery disease [9] ← [3] problems of modern obstetrics [8] 1 within a model of human diseases using Adcyap1-knockout mice, 3 in a model of human health using transgenic mice overexpressing 2 ADCYAP1 ← → [4] decreased fertility [10] [4] Adcyap1 within only pancreatic β-cells, ameliorated diabetes [11] 2 within a model of human diseases -

Improved and Efficient Therapy of Acromegaly by Implementation of a Personalized and Predictive Algorithm Including Molecular and Clinical Information
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Improved and efficient therapy of acromegaly by implementation of a personalized and predictive algorithm including molecular and clinical information PhD thesis by: Joan Gil Ortega Thesis supervisors: Prof. Manel Puig Domingo Dr. Mireia Jordà Ramos Tutor: Prof. Manel Puig Domingo Doctoral Program in Medicine. Department of Medicine. 2020 Acknowledgments M’agradaria agrair amb aquestes línies a les persones que han fet possible aquesta tesis, tant directament com indirectament. D’aquest període de la meva vida m’emporto moltes i bones experiències, grans aprenentatges i fins i tot, alguna nova habilitat. Però les persones que he trobat i m’han acompanyat durant aquests anys han estat el més important al·licient i el principal record que m’enduc d’aquests anys. I si parlem de persones no puc evitar anomenar, per contradictori que sembli, una institució, l’IGTP i l’antic IMPPC. On la primera persona que vaig conèixer va ser la meva directora de tesi Mireia Jordà que m’ha guiat durant tot aquest procés amb passió i dedicació. -

Prevalence of Human Growth Hormone-1 Gene Deletions Among Patients with Isolated Growth Hormone Deficiency from Different Populations
003 1-399819213105-0532$03.00/0 PEDIATRIC RESEARCH Vol. 3 1, No. 5, 1992 Copyright O 1992 international Pediatric Research Foundation, Inc. Prinled in U.S. A. Prevalence of Human Growth Hormone-1 Gene Deletions among Patients with Isolated Growth Hormone Deficiency from Different Populations P. E. MULLIS, A. AKINCI, CH. KANAKA, A. EBLE, AND C. G. D. BROOK Deparfment of Paediafrics, Inselspital, Bern, Switzerland [P.E.M.. Ch.K., A.E.]; Department of Paediatric Endocrinology, Dr. Sami Ulus Childrens Hospital, Ankara, Turkey [A.A.]; and Endocrine Unit, The Middlesex Hospital, London WIN 8AA. United Kingdom [C.G.D.B.] ABSTRACT. Familial isolated growth hormone deficiency suggested to be familial (2). Four distinct familial types of IGHD type IA results from homozygosity for either a 6.7-kb or a are well-differentiated on the basis of inheritance and other 7.6-kb hGH-1 gene deletion. Genomic DNA was extracted hormone deficiencies (3). One form is IGHD type IA, resulting from circulating lymphocytes of 78 subjects with severe from a GH-1 gene deletion (4, 5). Subjects with IGHD type IA isolated growth hormone deficiency (height < -4.5 SD may have short body length at birth and present occasionally score) and studied by polymerase chain amplification and with hypoglycemia, but severe growth retardation by 6 mo of by restriction endonuclease analysis looking for gene dele- age is a constant finding. Treatment with hGH is frequently tions within the hGH-gene cluster. The individuals ana- complicated by the development of anti-hGH antibodies in a lyzed were broadly grouped into three different populations titer sufficient to cause arrest ofresponse to the hGH replacement (North-European, n = 32; Mediterranean, n = 22; and (6).