Molecular Phylogenetic Analysis of Novel Spiroplasma Isolates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spiroplasma Arp Sequences: Relationships
SPIROPLASMA ARP SEQUENCES: RELATIONSHIPS WITH EXTRACHROMOSOMAL ELEMENTS By BHARAT DILIP JOSHI Bachelor of Science University of Pune, India 1995 Master of Science University of Pune, India 1997 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY July, 2006 SPIROPLASMA ARP SEQUENCES: RELATIONSHIPS WITH EXTRACHROMOSOMAL ELEMENTS Dissertation Approved: Dr. Ulrich K. Melcher Dissertation Adviser Dr. Andrew J. Mort Dr. Robert L. Matts Dr. Richard C. Essenberg ________________________________________________ Dr. Jacqueline Fletcher Dr. A. Gordon Emslie Dean of the Graduate College ii TABLE OF CONTENTS Chapter Page I. LITERATURE REVIEW ............................................................................... 1 Background ........................................................................................... 1 Economic Importance............................................................................ 1 Classification ......................................................................................... 2 Transmission in Nature ......................................................................... 3 Molecular Mollicute-host Interactions .................................................. 4 Molecular Spiroplasma-host Interactions ............................................. 4 Mollicute Extrachromosomal DNAs .................................................... 7 Objectives of the Present Study ........................................................... -
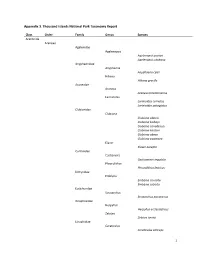
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Newsletter of the Biological Survey of Canada
Newsletter of the Biological Survey of Canada Vol. 40(1) Summer 2021 The Newsletter of the BSC is published twice a year by the In this issue Biological Survey of Canada, an incorporated not-for-profit From the editor’s desk............2 group devoted to promoting biodiversity science in Canada. Membership..........................3 President’s report...................4 BSC Facebook & Twitter...........5 Reminder: 2021 AGM Contributing to the BSC The Annual General Meeting will be held on June 23, 2021 Newsletter............................5 Reminder: 2021 AGM..............6 Request for specimens: ........6 Feature Articles: Student Corner 1. City Nature Challenge Bioblitz Shawn Abraham: New Student 2021-The view from 53.5 °N, Liaison for the BSC..........................7 by Greg Pohl......................14 Mayflies (mainlyHexagenia sp., Ephemeroptera: Ephemeridae): an 2. Arthropod Survey at Fort Ellice, MB important food source for adult by Robert E. Wrigley & colleagues walleye in NW Ontario lakes, by A. ................................................18 Ricker-Held & D.Beresford................8 Project Updates New book on Staphylinids published Student Corner by J. Klimaszewski & colleagues......11 New Student Liaison: Assessment of Chironomidae (Dip- Shawn Abraham .............................7 tera) of Far Northern Ontario by A. Namayandeh & D. Beresford.......11 Mayflies (mainlyHexagenia sp., Ephemerop- New Project tera: Ephemeridae): an important food source Help GloWorm document the distribu- for adult walleye in NW Ontario lakes, tion & status of native earthworms in by A. Ricker-Held & D.Beresford................8 Canada, by H.Proctor & colleagues...12 Feature Articles 1. City Nature Challenge Bioblitz Tales from the Field: Take me to the River, by Todd Lawton ............................26 2021-The view from 53.5 °N, by Greg Pohl..............................14 2. -

Diptera: Tabanidae) in Uruguay 2 Martín Lucasab; Tiago K
bioRxiv preprint doi: https://doi.org/10.1101/794479; this version posted October 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Diversity and seasonality of horse flies (Diptera: Tabanidae) in Uruguay 2 Martín Lucasab; Tiago K. Krolowc; Franklin Riet-Correaa; Antonio Thadeu M. Barrosd; 3 Rodrigo F. Krügere; Anderson Saraviaa; Cecilia Miraballesa* 4 5 a Instituto Nacional de Investigación Agropecuaria (INIA), Plataforma de Salud Animal, 6 Estación Experimental INIA Tacuarembó, Ruta 5 km 386, Tacuarembó, Uruguay, CP 7 45000. 8 b Facultad de Veterinaria, Universidad de la República, Alberto Lasplaces 1550, 9 Montevideo, Uruguay, CP 11600. 10 c Universidade Federal do Tocantins – UFT, Rua 03, Qd 17, S/N, Bairro Jardim dos 11 Ipês, Porto Nacional, TO, Brazil. 12 d Embrapa Gado de Corte, Campo Grande, MS, Brazil. 13 eUniversidade Federal de Pelotas, Instituto de Biologia, Departamento de Microbiologia 14 e Parasitologia, Campus Universitário Capão do Leão, s/nº, Pelotas, RS, Brazil. 15 ⁎ Corresponding author at: Instituto Nacional de Investigación Agropecuaria (INIA), 16 Plataforma de Salud Animal, Estación Experimental INIA Tacuarembó, Ruta 5 17 km 386, Tacuarembó, CP 45000, Uruguay. E-mail address: [email protected] (C. 18 Miraballes). 19 20 Abstract 21 Horse flies (Diptera: Tabanidae) are hematophagous insects that cause direct and 22 indirect losses in livestock production and are important vectors of pathogens. The aim 23 of this study was to determine the diversity and seasonality of horse fly species at an 24 experimental farm in Tacuarembó and the diversity of species in different departments 25 of Uruguay. -

Insects of Larose Forest (Excluding Lepidoptera and Odonates)
Insects of Larose Forest (Excluding Lepidoptera and Odonates) • Non-native species indicated by an asterisk* • Species in red are new for the region EPHEMEROPTERA Mayflies Baetidae Small Minnow Mayflies Baetidae sp. Small minnow mayfly Caenidae Small Squaregills Caenidae sp. Small squaregill Ephemerellidae Spiny Crawlers Ephemerellidae sp. Spiny crawler Heptageniiidae Flatheaded Mayflies Heptageniidae sp. Flatheaded mayfly Leptophlebiidae Pronggills Leptophlebiidae sp. Pronggill PLECOPTERA Stoneflies Perlodidae Perlodid Stoneflies Perlodid sp. Perlodid stonefly ORTHOPTERA Grasshoppers, Crickets and Katydids Gryllidae Crickets Gryllus pennsylvanicus Field cricket Oecanthus sp. Tree cricket Tettigoniidae Katydids Amblycorypha oblongifolia Angular-winged katydid Conocephalus nigropleurum Black-sided meadow katydid Microcentrum sp. Leaf katydid Scudderia sp. Bush katydid HEMIPTERA True Bugs Acanthosomatidae Parent Bugs Elasmostethus cruciatus Red-crossed stink bug Elasmucha lateralis Parent bug Alydidae Broad-headed Bugs Alydus sp. Broad-headed bug Protenor sp. Broad-headed bug Aphididae Aphids Aphis nerii Oleander aphid* Paraprociphilus tesselatus Woolly alder aphid Cicadidae Cicadas Tibicen sp. Cicada Cicadellidae Leafhoppers Cicadellidae sp. Leafhopper Coelidia olitoria Leafhopper Cuernia striata Leahopper Draeculacephala zeae Leafhopper Graphocephala coccinea Leafhopper Idiodonus kelmcottii Leafhopper Neokolla hieroglyphica Leafhopper 1 Penthimia americana Leafhopper Tylozygus bifidus Leafhopper Cercopidae Spittlebugs Aphrophora cribrata -

Field Guide to Western North American Fireflies
Field Guide to Western North American Fireflies By Larry Buschman (May 2015 Draft) Fireflies are also known as lightning bugs or glowworms. They are popular insects because they produce their own light (bioluminescence). They are not “flies” or “bugs” but beetles (order Coleoptera) with leathery first wings. Fireflies belong to the family “Lampyridae”. Identify members of this family as follows: a. They have an elongated body. b. The head telescopes in and out under the pronotum (the thoracic shield). c. The pronotum is usually large and shield- like. d. The pronotum often has colorful markings with yellow, tan, red, or orange pigment. Fig. 1. Photinus firefly e. Most species are 5-20 mm long. This Field Guide is intended for those who would like to identify the different fireflies in their environment. This guide covers the most common firefly species, but is not intended to be comprehensive. North America is blessed with several hundred species of Lampyrids—the firefly family. Many of them fly around flashing and are called “Fireflies” or “Lightning Bugs”. This Field Guide will focus on these fireflies. However, there are also some “Glowwarms” (Lampyrids that glow from the ground) and the “Dark Fireflies” (non-glowing Lampyrids). For research I am obliged to take voucher specimens. However, many populations are so small, especially in the west, that loosing even a few specimens can be expected to have negative effects on their populations. I would encourage most firefliers not to take specimens (practice catch and release) unless they will be preserved for science. Fireflies should not be collected by children to decorate their bodies etc—not in the west! How to Identify Fireflies Many fireflies can be identified by their flash patterns, but this is not as easy as it would seem. -

Coleoptera: Lampyridae)
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2020-03-23 Advances in the Systematics and Evolutionary Understanding of Fireflies (Coleoptera: Lampyridae) Gavin Jon Martin Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Life Sciences Commons BYU ScholarsArchive Citation Martin, Gavin Jon, "Advances in the Systematics and Evolutionary Understanding of Fireflies (Coleoptera: Lampyridae)" (2020). Theses and Dissertations. 8895. https://scholarsarchive.byu.edu/etd/8895 This Dissertation is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected]. Advances in the Systematics and Evolutionary Understanding of Fireflies (Coleoptera: Lampyridae) Gavin Jon Martin A dissertation submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Seth M. Bybee, Chair Marc A. Branham Jamie L. Jensen Kathrin F. Stanger-Hall Michael F. Whiting Department of Biology Brigham Young University Copyright © 2020 Gavin Jon Martin All Rights Reserved ABSTRACT Advances in the Systematics and Evolutionary Understanding of Fireflies (Coleoptera: Lampyridae) Gavin Jon Martin Department of Biology, BYU Doctor of Philosophy Fireflies are a cosmopolitan group of bioluminescent beetles classified in the family Lampyridae. The first catalogue of Lampyridae was published in 1907 and since that time, the classification and systematics of fireflies have been in flux. Several more recent catalogues and classification schemes have been published, but rarely have they taken phylogenetic history into account. Here I infer the first large scale anchored hybrid enrichment phylogeny for the fireflies and use this phylogeny as a backbone to inform classification. -

Diversity and Seasonality of Horse Flies (Diptera: Tabanidae) in Uruguay 2 Martín Lucasab; Tiago K
bioRxiv preprint doi: https://doi.org/10.1101/794479; this version posted October 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Diversity and seasonality of horse flies (Diptera: Tabanidae) in Uruguay 2 Martín Lucasab; Tiago K. Krolowc; Franklin Riet-Correaa; Antonio Thadeu M. Barrosd; 3 Rodrigo F. Krügere; Anderson Saraviaa; Cecilia Miraballesa* 4 5 a Instituto Nacional de Investigación Agropecuaria (INIA), Plataforma de Salud Animal, 6 Estación Experimental INIA Tacuarembó, Ruta 5 km 386, Tacuarembó, Uruguay, CP 7 45000. 8 b Facultad de Veterinaria, Universidad de la República, Alberto Lasplaces 1550, 9 Montevideo, Uruguay, CP 11600. 10 c Universidade Federal do Tocantins – UFT, Rua 03, Qd 17, S/N, Bairro Jardim dos 11 Ipês, Porto Nacional, TO, Brazil. 12 d Embrapa Gado de Corte, Campo Grande, MS, Brazil. 13 eUniversidade Federal de Pelotas, Instituto de Biologia, Departamento de Microbiologia 14 e Parasitologia, Campus Universitário Capão do Leão, s/nº, Pelotas, RS, Brazil. 15 ⁎ Corresponding author at: Instituto Nacional de Investigación Agropecuaria (INIA), 16 Plataforma de Salud Animal, Estación Experimental INIA Tacuarembó, Ruta 5 17 km 386, Tacuarembó, CP 45000, Uruguay. E-mail address: [email protected] (C. 18 Miraballes). 19 20 Abstract 21 Horse flies (Diptera: Tabanidae) are hematophagous insects that cause direct and 22 indirect losses in livestock production and are important vectors of pathogens. The aim 23 of this study was to determine the diversity and seasonality of horse fly species at an 24 experimental farm in Tacuarembó and the diversity of species in different departments 25 of Uruguay. -

Diversity and Seasonality of Horse Flies (Diptera: Tabanidae) in Uruguay
www.nature.com/scientificreports OPEN Diversity and seasonality of horse fies (Diptera: Tabanidae) in Uruguay Martín Lucas1,2, Tiago K. Krolow3, Franklin Riet-Correa1, Antonio Thadeu M. Barros4, Rodrigo F. Krüger5, Anderson Saravia1 & Cecilia Miraballes 1* Horse fies (Diptera: Tabanidae) cause direct and indirect losses in livestock production and are important vectors of pathogens. The aim of this study was to determine the diversity and seasonality of horse fy species at an experimental farm in Tacuarembó and the diversity of species in diferent departments of Uruguay. For 20 months, systematic collections were performed in two diferent environments at the experimental farm using Nzi and Malaise traps. In addition, nonsystematic collections were performed at farms located in the departments of Paysandú, Tacuarembó and Colonia. A total of 3,666 horse fies were collected, and 16 species were identifed. These species included three species that had not been previously recorded in Uruguay, namely, Dasybasis ornatissima (Brèthes), Dasybasis missionum (Macquart), and Tabanus af. platensis Brèthes, and a species that had not been previously taxonomically described (Tabanus sp.1). Among the systematically captured samples, the most abundant species were Tabanus campestris Brèthes, T. af. platensis and D. missionum, representing 77.6% of the collected specimens. The horse fy season in Tacuarembó started in September and ended in May. No horse fies were caught during winter. Variations in the prevalences of species in the diferent departments were observed, which indicates the need for new sampling eforts. Horse fies (Tabanidae) are hematophagous dipterans that cause direct losses to livestock production due to irri- tation, stress, and blood loss in animals, particularly cattle and horses1. -

Powell Mountain Karst Preserve: Biological Inventory of Vegetation Communities, Vascular Plants, and Selected Animal Groups
Powell Mountain Karst Preserve: Biological Inventory of Vegetation Communities, Vascular Plants, and Selected Animal Groups Final Report Prepared by: Christopher S. Hobson For: The Cave Conservancy of the Virginias Date: 15 April 2010 This report may be cited as follows: Hobson, C.S. 2010. Powell Mountain Karst Preserve: Biological Inventory of Vegetation Communities, Vascular Plants, and Selected Animal Groups. Natural Heritage Technical Report 10-12. Virginia Department of Conservation and Recreation, Division of Natural Heritage, Richmond, Virginia. Unpublished report submitted to The Cave Conservancy of the Virginias. April 2010. 30 pages plus appendices. COMMONWEALTH of VIRGINIA Biological Inventory of Vegetation Communities, Vascular Plants, and Selected Animal Groups Virginia Department of Conservation and Recreation Division of Natural Heritage Natural Heritage Technical Report 10-12 April 2010 Contents List of Tables......................................................................................................................... ii List of Figures........................................................................................................................ iii Introduction............................................................................................................................ 1 Geology.................................................................................................................................. 2 Explanation of the Natural Heritage Ranking System.......................................................... -

Neotropical Diptera 16: 1-199 (April 15, 2009) Depto
Coscarón & Papavero Neotropical Diptera Neotropical Diptera 16: 1-199 (April 15, 2009) Depto. de Biologia - FFCLRP ISSN 1982-7121 Universidade de São Paulo www.neotropicaldiptera.org Ribeirão Preto, SP, Brazil Catalogue of Neotropical Diptera. Tabanidae1 Sixto Coscarón Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del Bosque, 1900 La Plata, República Argentina Pesquisador Visitante do Departamento de Biologia, Faculdade de Filosofia, Ciências e Letras, Universidade de São Paulo, Ribeirão Preto, SP, Brasil e-mail: [email protected] & Nelson Papavero Museu de Zoologia, Universidade de São Paulo, São Paulo, SP, Brasil Pesquisador Visitante do Departamento de Biologia, Faculdade de Filosofia, Ciências e Letras, Universidade de São Paulo, Ribeirão Preto, SP, Brasil Introduction This catalogue includes 1082 nominal species, distributed as in the table below (plus 34 unrecognized ones), 28 nomina nuda and 809 references. Subfamily Tribe Genus Subgenus Number of species CHRYSOPSINAE 88 Bouvieromyiini 3 Pseudotabanus Coracella 3 Chrysopsini 93 Chrysops 75 Silvius 9 Assipala 5 Griseosilvius 3 Silvius 1 Rhinomyzini 1 Betrequia 1 PANGONIINAE 315 Mycteromyiini 16 Mycteromyia 3 Promycteromyia 9 Silvestriellus 4 1 This project was supported by FAPESP grants # 2003/10.274-9, 2007/50877-5, and 2007/50878-1. Neotropical Diptera 16 1 Catalogue of the Neotropical Diptera. Tabanidae Pangoniini 129 Apatolestes 5 Apotolestes 4 Lanellus 1 Archeomyotes 1 Austromyans 1 Boliviamyia 1 Brennania 1 Chaetopalpus 1 Esenbeckia -

Neotropical Diptera
Coscarón & Papavero Neotropical Diptera Neotropical Diptera 6: 1-137 (April 15, 2009) Depto. de Biologia - FFCLRP ISSN 1982-7121 Universidade de São Paulo www.neotropicaldiptera.org Ribeirão Preto, SP, Brazil Manual of Neotropical Diptera. Tabanidae1 Sixto Coscarón Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del Bosque, 1900 La Plata, República Argentina Pesquisador Visitante do Departamento de Biologia, Faculdade de Filosofia, Ciências e Letras, Universidade de São Paulo, Ribeirão Preto, SP, Brasil e-mail: [email protected] & Nelson Papavero Museu de Zoologia, Universidade de São Paulo, São Paulo, SP, Brasil Pesquisador Visitante do Departamento de Biologia, Faculdade de Filosofia, Ciências e Letras, Universidade de São Paulo, Ribeirão Preto, SP, Brasil Introduction According to Fairchild (1981: 290-291): “Adult tabanids are found in almost all conceivable habitats, from salt lakes and ocean beaches to snowline in the Andes, and from the extreme deserts of coastal Peru and Chile to the nearly perpetually rainy cloud forests of many parts of central America and the eastern slopes of the Andes. With the exception of about a dozen common and wide-ranging species which seem to prefer the more open and disturbed habiats created by man’s agricultural activities, most tabanids have definite habitat preferences. This aspect of their biolgy has been little studied, although Fairchild (1953) summarized knowledge of the arboreal species and Chvala & Stary (1967) noted habitat referencs for Cuban speces. Philip (1978) and Fairchild (1973) noted preferences of certain species for the mangrove swamp habitat [Lutz, 1922: 148 had already noted that larvae of Tabanus obsoletus Wiedemann, 1821 live in that environment].