2983 Spiroplasmas: Evolutionary Relationships and Biodiversity Laura B. Regassa 1 and Gail E. Gasparich 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spiroplasma Arp Sequences: Relationships
SPIROPLASMA ARP SEQUENCES: RELATIONSHIPS WITH EXTRACHROMOSOMAL ELEMENTS By BHARAT DILIP JOSHI Bachelor of Science University of Pune, India 1995 Master of Science University of Pune, India 1997 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY July, 2006 SPIROPLASMA ARP SEQUENCES: RELATIONSHIPS WITH EXTRACHROMOSOMAL ELEMENTS Dissertation Approved: Dr. Ulrich K. Melcher Dissertation Adviser Dr. Andrew J. Mort Dr. Robert L. Matts Dr. Richard C. Essenberg ________________________________________________ Dr. Jacqueline Fletcher Dr. A. Gordon Emslie Dean of the Graduate College ii TABLE OF CONTENTS Chapter Page I. LITERATURE REVIEW ............................................................................... 1 Background ........................................................................................... 1 Economic Importance............................................................................ 1 Classification ......................................................................................... 2 Transmission in Nature ......................................................................... 3 Molecular Mollicute-host Interactions .................................................. 4 Molecular Spiroplasma-host Interactions ............................................. 4 Mollicute Extrachromosomal DNAs .................................................... 7 Objectives of the Present Study ........................................................... -
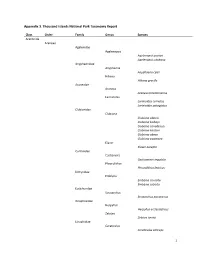
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Newsletter of the Biological Survey of Canada
Newsletter of the Biological Survey of Canada Vol. 40(1) Summer 2021 The Newsletter of the BSC is published twice a year by the In this issue Biological Survey of Canada, an incorporated not-for-profit From the editor’s desk............2 group devoted to promoting biodiversity science in Canada. Membership..........................3 President’s report...................4 BSC Facebook & Twitter...........5 Reminder: 2021 AGM Contributing to the BSC The Annual General Meeting will be held on June 23, 2021 Newsletter............................5 Reminder: 2021 AGM..............6 Request for specimens: ........6 Feature Articles: Student Corner 1. City Nature Challenge Bioblitz Shawn Abraham: New Student 2021-The view from 53.5 °N, Liaison for the BSC..........................7 by Greg Pohl......................14 Mayflies (mainlyHexagenia sp., Ephemeroptera: Ephemeridae): an 2. Arthropod Survey at Fort Ellice, MB important food source for adult by Robert E. Wrigley & colleagues walleye in NW Ontario lakes, by A. ................................................18 Ricker-Held & D.Beresford................8 Project Updates New book on Staphylinids published Student Corner by J. Klimaszewski & colleagues......11 New Student Liaison: Assessment of Chironomidae (Dip- Shawn Abraham .............................7 tera) of Far Northern Ontario by A. Namayandeh & D. Beresford.......11 Mayflies (mainlyHexagenia sp., Ephemerop- New Project tera: Ephemeridae): an important food source Help GloWorm document the distribu- for adult walleye in NW Ontario lakes, tion & status of native earthworms in by A. Ricker-Held & D.Beresford................8 Canada, by H.Proctor & colleagues...12 Feature Articles 1. City Nature Challenge Bioblitz Tales from the Field: Take me to the River, by Todd Lawton ............................26 2021-The view from 53.5 °N, by Greg Pohl..............................14 2. -

Diptera: Tabanidae) in Uruguay 2 Martín Lucasab; Tiago K
bioRxiv preprint doi: https://doi.org/10.1101/794479; this version posted October 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Diversity and seasonality of horse flies (Diptera: Tabanidae) in Uruguay 2 Martín Lucasab; Tiago K. Krolowc; Franklin Riet-Correaa; Antonio Thadeu M. Barrosd; 3 Rodrigo F. Krügere; Anderson Saraviaa; Cecilia Miraballesa* 4 5 a Instituto Nacional de Investigación Agropecuaria (INIA), Plataforma de Salud Animal, 6 Estación Experimental INIA Tacuarembó, Ruta 5 km 386, Tacuarembó, Uruguay, CP 7 45000. 8 b Facultad de Veterinaria, Universidad de la República, Alberto Lasplaces 1550, 9 Montevideo, Uruguay, CP 11600. 10 c Universidade Federal do Tocantins – UFT, Rua 03, Qd 17, S/N, Bairro Jardim dos 11 Ipês, Porto Nacional, TO, Brazil. 12 d Embrapa Gado de Corte, Campo Grande, MS, Brazil. 13 eUniversidade Federal de Pelotas, Instituto de Biologia, Departamento de Microbiologia 14 e Parasitologia, Campus Universitário Capão do Leão, s/nº, Pelotas, RS, Brazil. 15 ⁎ Corresponding author at: Instituto Nacional de Investigación Agropecuaria (INIA), 16 Plataforma de Salud Animal, Estación Experimental INIA Tacuarembó, Ruta 5 17 km 386, Tacuarembó, CP 45000, Uruguay. E-mail address: [email protected] (C. 18 Miraballes). 19 20 Abstract 21 Horse flies (Diptera: Tabanidae) are hematophagous insects that cause direct and 22 indirect losses in livestock production and are important vectors of pathogens. The aim 23 of this study was to determine the diversity and seasonality of horse fly species at an 24 experimental farm in Tacuarembó and the diversity of species in different departments 25 of Uruguay. -

Metazoan Ribosome Inactivating Protein Encoding Genes Acquired by Horizontal Gene Transfer Received: 30 September 2016 Walter J
www.nature.com/scientificreports OPEN Metazoan Ribosome Inactivating Protein encoding genes acquired by Horizontal Gene Transfer Received: 30 September 2016 Walter J. Lapadula1, Paula L. Marcet2, María L. Mascotti1, M. Virginia Sanchez-Puerta3 & Accepted: 5 April 2017 Maximiliano Juri Ayub1 Published: xx xx xxxx Ribosome inactivating proteins (RIPs) are RNA N-glycosidases that depurinate a specific adenine residue in the conserved sarcin/ricin loop of 28S rRNA. These enzymes are widely distributed among plants and their presence has also been confirmed in several bacterial species. Recently, we reported for the first timein silico evidence of RIP encoding genes in metazoans, in two closely related species of insects: Aedes aegypti and Culex quinquefasciatus. Here, we have experimentally confirmed the presence of these genes in mosquitoes and attempted to unveil their evolutionary history. A detailed study was conducted, including evaluation of taxonomic distribution, phylogenetic inferences and microsynteny analyses, indicating that mosquito RIP genes derived from a single Horizontal Gene Transfer (HGT) event, probably from a cyanobacterial donor species. Moreover, evolutionary analyses show that, after the HGT event, these genes evolved under purifying selection, strongly suggesting they play functional roles in these organisms. Ribosome inactivating proteins (RIPs, EC 3.2.2.22) irreversibly modify ribosomes through the depurination of an adenine residue in the conserved alpha-sarcin/ricin loop of 28S rRNA1–4. This modification prevents the binding of elongation factor 2 to the ribosome, arresting protein synthesis5, 6. The occurrence of RIP genes has been exper- imentally confirmed in a wide range of plant taxa, as well as in several species of Gram positive and Gram negative bacteria7–9. -

Factors Affecting Cuticular Hydrocarbon
FACTORS AFFECTING CUTICULAR HYDROCARBON ANALYSIS FOR IDENTIFICATION OF FIELD POPULATIONS OF TABANUS MULARIS STONE (DIPTERA: TABANIDAE) IN OKLAHOMA By ROBERT A. MIHALOVICH Bachelor of Arts Washington and Jefferson College Washington, Pennsylvania 1993 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE May, 1996 FACTORS AFFECTING CUTICULAR HYDROCARBON ANALYSIS FOR IDENTIFICATION OF FIELD POPULATIONS OF TABANUS MULARIS STONE (DIPTERA: TABANIDAE) IN OKLAHOMA Thesis Approved: Dean of the Graduate College ii ACKNOWLEDGEMENTS I would like to express my sincere thanks to Dr. Russell Wright and the Department of Entomology for.their generous support throughout the course of this project. I also thank Dr. Richard Berberet for serving as a member of my committee. Very special thanks goes to Dr. Jack DiUwith for his invaluable assistance and to Dr. William Warde and the late Dr. W. Scott Fargo for helping me with the statistical analysis. I also express my appreciation to Lisa Cobum and Richard Grantham for all of their valuable time and assistance. A very special word of thanks goes to Sarah McLean for her thoughtfulness, support and data processing ability. Most of all, my deepest appreciation goes to my parents Robert and Barbara and my family and friends who supported me throughout this study. I dedicate this work to the memory of my grandfather Slim, and my uncle Nick who were always an inspiration !o me. iii TABLE OF CONTENTS Chapter Page I. INTRODUCTION.................................................... 1 II. LITERATURE REVIEW. ...... .. .......... .. ...... ... ............... .. 3 Tabanus mularis Complex. ................................. 3 Cuticular Hydrocarbons. -

Insects of Larose Forest (Excluding Lepidoptera and Odonates)
Insects of Larose Forest (Excluding Lepidoptera and Odonates) • Non-native species indicated by an asterisk* • Species in red are new for the region EPHEMEROPTERA Mayflies Baetidae Small Minnow Mayflies Baetidae sp. Small minnow mayfly Caenidae Small Squaregills Caenidae sp. Small squaregill Ephemerellidae Spiny Crawlers Ephemerellidae sp. Spiny crawler Heptageniiidae Flatheaded Mayflies Heptageniidae sp. Flatheaded mayfly Leptophlebiidae Pronggills Leptophlebiidae sp. Pronggill PLECOPTERA Stoneflies Perlodidae Perlodid Stoneflies Perlodid sp. Perlodid stonefly ORTHOPTERA Grasshoppers, Crickets and Katydids Gryllidae Crickets Gryllus pennsylvanicus Field cricket Oecanthus sp. Tree cricket Tettigoniidae Katydids Amblycorypha oblongifolia Angular-winged katydid Conocephalus nigropleurum Black-sided meadow katydid Microcentrum sp. Leaf katydid Scudderia sp. Bush katydid HEMIPTERA True Bugs Acanthosomatidae Parent Bugs Elasmostethus cruciatus Red-crossed stink bug Elasmucha lateralis Parent bug Alydidae Broad-headed Bugs Alydus sp. Broad-headed bug Protenor sp. Broad-headed bug Aphididae Aphids Aphis nerii Oleander aphid* Paraprociphilus tesselatus Woolly alder aphid Cicadidae Cicadas Tibicen sp. Cicada Cicadellidae Leafhoppers Cicadellidae sp. Leafhopper Coelidia olitoria Leafhopper Cuernia striata Leahopper Draeculacephala zeae Leafhopper Graphocephala coccinea Leafhopper Idiodonus kelmcottii Leafhopper Neokolla hieroglyphica Leafhopper 1 Penthimia americana Leafhopper Tylozygus bifidus Leafhopper Cercopidae Spittlebugs Aphrophora cribrata -

Complete Genomes of Two Dipteran-Associated Spiroplasmas Provided Insights Into the Origin, Dynamics, and Impacts of Viral Invasion in Spiroplasma
GBE Complete Genomes of Two Dipteran-Associated Spiroplasmas Provided Insights into the Origin, Dynamics, and Impacts of Viral Invasion in Spiroplasma Chuan Ku1,Wen-SuiLo1,2,3, Ling-Ling Chen1, and Chih-Horng Kuo1,2,4,* 1Institute of Plant and Microbial Biology, Academia Sinica, Taipei, Taiwan 2Molecular and Biological Agricultural Sciences Program, Taiwan International Graduate Program, National Chung Hsing University and Academia Sinica, Taipei, Taiwan 3Graduate Institute of Biotechnology, National Chung Hsing University, Taichung, Taiwan 4Biotechnology Center, National Chung Hsing University, Taichung, Taiwan *Corresponding author: E-mail: [email protected]. Accepted: May 21, 2013 Data deposition: The genome sequences reported in this study have been deposited at DDBJ/EMBL/GenBank under the accessions CP005077 and CP005078. Abstract Spiroplasma is a genus of wall-less, low-GC, Gram-positive bacteria with helical morphology. As commensals or pathogens of plants, insects, ticks, or crustaceans, they are closely related with mycoplasmas and form a monophyletic group (Spiroplasma– Entomoplasmataceae–Mycoides) with Mycoplasma mycoides and its relatives. In this study, we report the complete genome sequences of Spiroplasma chrysopicola and S. syrphidicola from the Chrysopicola clade. These species form the sister group to the Citri clade, which includes several well-known pathogenic spiroplasmas. Surprisingly, these two newly available genomes from the Chrysopicola clade contain no plectroviral genes, which were found to be highly repetitive in the previously sequenced genomes from the Citri clade. Based on the genome alignment and patterns of GC-skew, these two Chrysopicola genomes appear to be relatively stable, rather than being highly rearranged as those from the Citri clade. -

Field Guide to Western North American Fireflies
Field Guide to Western North American Fireflies By Larry Buschman (May 2015 Draft) Fireflies are also known as lightning bugs or glowworms. They are popular insects because they produce their own light (bioluminescence). They are not “flies” or “bugs” but beetles (order Coleoptera) with leathery first wings. Fireflies belong to the family “Lampyridae”. Identify members of this family as follows: a. They have an elongated body. b. The head telescopes in and out under the pronotum (the thoracic shield). c. The pronotum is usually large and shield- like. d. The pronotum often has colorful markings with yellow, tan, red, or orange pigment. Fig. 1. Photinus firefly e. Most species are 5-20 mm long. This Field Guide is intended for those who would like to identify the different fireflies in their environment. This guide covers the most common firefly species, but is not intended to be comprehensive. North America is blessed with several hundred species of Lampyrids—the firefly family. Many of them fly around flashing and are called “Fireflies” or “Lightning Bugs”. This Field Guide will focus on these fireflies. However, there are also some “Glowwarms” (Lampyrids that glow from the ground) and the “Dark Fireflies” (non-glowing Lampyrids). For research I am obliged to take voucher specimens. However, many populations are so small, especially in the west, that loosing even a few specimens can be expected to have negative effects on their populations. I would encourage most firefliers not to take specimens (practice catch and release) unless they will be preserved for science. Fireflies should not be collected by children to decorate their bodies etc—not in the west! How to Identify Fireflies Many fireflies can be identified by their flash patterns, but this is not as easy as it would seem. -

Thermal Sensitivity of the Spiroplasma-Drosophila Hydei Protective Symbiosis: the Best of 2 Climes, the Worst of Climes
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.30.070938; this version posted May 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Thermal sensitivity of the Spiroplasma-Drosophila hydei protective symbiosis: The best of 2 climes, the worst of climes. 3 4 Chris Corbin, Jordan E. Jones, Ewa Chrostek, Andy Fenton & Gregory D. D. Hurst* 5 6 Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Crown 7 Street, Liverpool L69 7ZB, UK 8 9 * For correspondence: [email protected] 10 11 Short title: Thermal sensitivity of a protective symbiosis 12 13 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.04.30.070938; this version posted May 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 14 Abstract 15 16 The outcome of natural enemy attack in insects has commonly been found to be influenced 17 by the presence of protective symbionts in the host. The degree to which protection 18 functions in natural populations, however, will depend on the robustness of the phenotype 19 to variation in the abiotic environment. We studied the impact of a key environmental 20 parameter – temperature – on the efficacy of the protective effect of the symbiont 21 Spiroplasma on its host Drosophila hydei, against attack by the parasitoid wasp Leptopilina 22 heterotoma. -

Diptera) in East Africa
A peer-reviewed open-access journal ZooKeys 769: 117–144Morphological (2018) re-description and molecular identification of Tabanidae... 117 doi: 10.3897/zookeys.769.21144 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Morphological re-description and molecular identification of Tabanidae (Diptera) in East Africa Claire M. Mugasa1,2, Jandouwe Villinger1, Joseph Gitau1, Nelly Ndungu1,3, Marc Ciosi1,4, Daniel Masiga1 1 International Centre of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya 2 School of Biosecurity Biotechnical Laboratory Sciences, College of Veterinary Medicine, Animal Resources and Biosecurity (COVAB), Makerere University, Kampala, Uganda 3 Social Insects Research Group, Department of Zoology and Entomo- logy, University of Pretoria, Hatfield, 0028 Pretoria, South Africa 4 Institute of Molecular Cell and Systems Biology, University of Glasgow, Glasgow, UK Corresponding author: Daniel Masiga ([email protected]) Academic editor: T. Dikow | Received 19 October 2017 | Accepted 9 April 2018 | Published 26 June 2018 http://zoobank.org/AB4EED07-0C95-4020-B4BB-E6EEE5AC8D02 Citation: Mugasa CM, Villinger J, Gitau J, Ndungu N, Ciosi M, Masiga D (2018) Morphological re-description and molecular identification of Tabanidae (Diptera) in East Africa. ZooKeys 769: 117–144.https://doi.org/10.3897/ zookeys.769.21144 Abstract Biting flies of the family Tabanidae are important vectors of human and animal diseases across conti- nents. However, records of Africa tabanids are fragmentary and mostly cursory. To improve identification, documentation and description of Tabanidae in East Africa, a baseline survey for the identification and description of Tabanidae in three eastern African countries was conducted. Tabanids from various loca- tions in Uganda (Wakiso District), Tanzania (Tarangire National Park) and Kenya (Shimba Hills National Reserve, Muhaka, Nguruman) were collected. -

Studies on Vision and Visual Attraction of the Salt Marsh Horse Fly, Tabanus Nigrovittatus Macquart
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations 1896 - February 2014 1-1-1984 Studies on vision and visual attraction of the salt marsh horse fly, Tabanus nigrovittatus Macquart. Sandra Anne Allan University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_1 Recommended Citation Allan, Sandra Anne, "Studies on vision and visual attraction of the salt marsh horse fly, Tabanus nigrovittatus Macquart." (1984). Doctoral Dissertations 1896 - February 2014. 5625. https://scholarworks.umass.edu/dissertations_1/5625 This Open Access Dissertation is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations 1896 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. STUDIES ON VISION AND VISUAL ATTRACTION OF THE SALT MARSH HORSE FLY, TABANUS NIGROVITTATUS MACQUART A Dissertation Presented By SANDRA ANNE ALLAN Submittted to the Graduate School of the University of Massachusetts in partial fulfillment of the requirements of the degree of DOCTOR OF PHILOSOPHY February 1984 Department of Entomology *> Sandra Anne Allan All Rights Reserved ii STUDIES ON VISION AND VISUAL ATTRACTION OF THE SALT MARSH HORSE FLY, TABANUS NIGROVITTATUS MACQUART A Dissertation Presented By SANDRA ANNE ALLAN Approved as to style and content by: iii DEDICATION In memory of my grandparents who have provided inspiration ACKNOWLEDGEMENTS I wish to express my most sincere appreciation to my advisor. Dr. John G. Stoffolano, Jr. for his guidance, constructive criticism and suggestions. I would also like to thank him for allowing me to pursue my research with a great deal of independence which has allowed me to develop as a scientist and as a person.