Coleoptera: Lampyridae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beetle Appreciation Diversity and Classification of Common Beetle Families Christopher E
Beetle Appreciation Diversity and Classification of Common Beetle Families Christopher E. Carlton Louisiana State Arthropod Museum Coleoptera Families Everyone Should Know (Checklist) Suborder Adephaga Suborder Polyphaga, cont. •Carabidae Superfamily Scarabaeoidea •Dytiscidae •Lucanidae •Gyrinidae •Passalidae Suborder Polyphaga •Scarabaeidae Superfamily Staphylinoidea Superfamily Buprestoidea •Ptiliidae •Buprestidae •Silphidae Superfamily Byrroidea •Staphylinidae •Heteroceridae Superfamily Hydrophiloidea •Dryopidae •Hydrophilidae •Elmidae •Histeridae Superfamily Elateroidea •Elateridae Coleoptera Families Everyone Should Know (Checklist, cont.) Suborder Polyphaga, cont. Suborder Polyphaga, cont. Superfamily Cantharoidea Superfamily Cucujoidea •Lycidae •Nitidulidae •Cantharidae •Silvanidae •Lampyridae •Cucujidae Superfamily Bostrichoidea •Erotylidae •Dermestidae •Coccinellidae Bostrichidae Superfamily Tenebrionoidea •Anobiidae •Tenebrionidae Superfamily Cleroidea •Mordellidae •Cleridae •Meloidae •Anthicidae Coleoptera Families Everyone Should Know (Checklist, cont.) Suborder Polyphaga, cont. Superfamily Chrysomeloidea •Chrysomelidae •Cerambycidae Superfamily Curculionoidea •Brentidae •Curculionidae Total: 35 families of 131 in the U.S. Suborder Adephaga Family Carabidae “Ground and Tiger Beetles” Terrestrial predators or herbivores (few). 2600 N. A. spp. Suborder Adephaga Family Dytiscidae “Predacious diving beetles” Adults and larvae aquatic predators. 500 N. A. spp. Suborder Adephaga Family Gyrindae “Whirligig beetles” Aquatic, on water -

An Annotated Checklist of Wisconsin Handsome Fungus Beetles (Coleoptera: Endomychidae)
The Great Lakes Entomologist Volume 40 Numbers 3 & 4 - Fall/Winter 2007 Numbers 3 & Article 9 4 - Fall/Winter 2007 October 2007 An Annotated Checklist of Wisconsin Handsome Fungus Beetles (Coleoptera: Endomychidae) Michele B. Price University of Wisconsin Daniel K. Young University of Wisconsin Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Price, Michele B. and Young, Daniel K. 2007. "An Annotated Checklist of Wisconsin Handsome Fungus Beetles (Coleoptera: Endomychidae)," The Great Lakes Entomologist, vol 40 (2) Available at: https://scholar.valpo.edu/tgle/vol40/iss2/9 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Price and Young: An Annotated Checklist of Wisconsin Handsome Fungus Beetles (Cole 2007 THE GREAT LAKES ENTOMOLOGIST 177 AN Annotated Checklist of Wisconsin Handsome Fungus Beetles (Coleoptera: Endomychidae) Michele B. Price1 and Daniel K. Young1 ABSTRACT The first comprehensive survey of Wisconsin Endomychidae was initiated in 1998. Throughout Wisconsin sampling sites were selected based on habitat type and sampling history. Wisconsin endomychids were hand collected from fungi and under tree bark; successful trapping methods included cantharidin- baited pitfall traps, flight intercept traps, and Lindgren funnel traps. Examina- tion of literature records, museum and private collections, and field research yielded 10 species, three of which are new state records. Two dubious records, Epipocus unicolor Horn and Stenotarsus hispidus (Herbst), could not be con- firmed. -
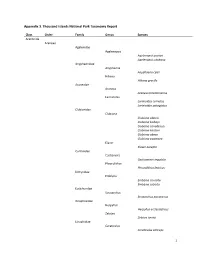
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Coleoptera: Endomychidae: Leiestinae) with a Checklist and Nomenclatural Notes Regarding Fossil Endomychidae
Zootaxa 3755 (4): 391–400 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3755.4.5 http://zoobank.org/urn:lsid:zoobank.org:pub:13446D49-76A1-4C12-975E-F59106AF4BD3 Glesirhanis bercioi, a new genus and species from Baltic amber (Coleoptera: Endomychidae: Leiestinae) with a checklist and nomenclatural notes regarding fossil Endomychidae FLOYD W. SHOCKLEY1& VITALY I. ALEKSEEV2 1Department of Entomology, National Museum of Natural History, Smithsonian Institution, P.O. Box 37012, MRC 165, Washington, DC 20013-7012, U.S.A. Email: [email protected] 2Department of Zootechny, Kaliningrad State Technical University, Sovetsky av. 1. 236000, Kaliningrad, Russia. E-mail: [email protected] Abstract A new genus and species of handsome fungus beetle, Glesirhanis bercioi gen. nov., sp. nov. (Coleoptera: Endomychidae: Leiestinae) is described from Baltic amber. The newly described genus is compared with all known extant and extinct genera of the subfamily. A key to the genera of Leiestinae including fossils and a checklist of fossil Endomychidae are provided. The status of two taxa previously placed in Endomychidae, Palaeoendomychus gymnus Zhang and Tetrameropsis mesozoica Kirejtshuk & Azar, is discussed, and a new status for the latter, elevating it to the family-level as Tetrameropseidae status nov., is proposed. Key words: new genus, new species, new status, Coleoptera, Endomychidae, Leiestinae, Baltic amber, Tertiary, Eocene, key, checklist, fossil Introduction Baltic amber (succinite) constitutes the largest known deposit of fossil plant resin and the richest repository of fossil insects of any age. Unfortunately, most references to Coleoptera in Baltic amber are only determined to family or generic levels. -

Newsletter of the Biological Survey of Canada
Newsletter of the Biological Survey of Canada Vol. 40(1) Summer 2021 The Newsletter of the BSC is published twice a year by the In this issue Biological Survey of Canada, an incorporated not-for-profit From the editor’s desk............2 group devoted to promoting biodiversity science in Canada. Membership..........................3 President’s report...................4 BSC Facebook & Twitter...........5 Reminder: 2021 AGM Contributing to the BSC The Annual General Meeting will be held on June 23, 2021 Newsletter............................5 Reminder: 2021 AGM..............6 Request for specimens: ........6 Feature Articles: Student Corner 1. City Nature Challenge Bioblitz Shawn Abraham: New Student 2021-The view from 53.5 °N, Liaison for the BSC..........................7 by Greg Pohl......................14 Mayflies (mainlyHexagenia sp., Ephemeroptera: Ephemeridae): an 2. Arthropod Survey at Fort Ellice, MB important food source for adult by Robert E. Wrigley & colleagues walleye in NW Ontario lakes, by A. ................................................18 Ricker-Held & D.Beresford................8 Project Updates New book on Staphylinids published Student Corner by J. Klimaszewski & colleagues......11 New Student Liaison: Assessment of Chironomidae (Dip- Shawn Abraham .............................7 tera) of Far Northern Ontario by A. Namayandeh & D. Beresford.......11 Mayflies (mainlyHexagenia sp., Ephemerop- New Project tera: Ephemeridae): an important food source Help GloWorm document the distribu- for adult walleye in NW Ontario lakes, tion & status of native earthworms in by A. Ricker-Held & D.Beresford................8 Canada, by H.Proctor & colleagues...12 Feature Articles 1. City Nature Challenge Bioblitz Tales from the Field: Take me to the River, by Todd Lawton ............................26 2021-The view from 53.5 °N, by Greg Pohl..............................14 2. -

Methodologies for Monitoring Fireflies in Hong Kong
40 Methodologies for monitoring fireflies Methodologies for monitoring fireflies in Hong Kong Yiu Vor 31E, Tin Sam Tsuen, Kam Sheung Road, Yuen Long, N.T., Hong Kong. Email: [email protected] ABSTRACT record fireflies qualitatively and quantitatively, including: In total 241 field visits to 47 different sites in Hong Kong a. Malaise traps. Ten traps were set for a general were conducted specifically for firefly survey, from 2009 insects study in 2014 and small quantity of fireflies to 2020. Various methods were used to record fireflies were collected; qualitatively and quantitatively. Local restrictedness of 29 species of Hong Kong fireflies are listed. Methods b. Quadrat count and point count. Used in high for accessing the population of different firefly species visibility areas with concentration of flying fireflies are discussed and recommended according to their displaying light at night. Area of the quadrats was distribution characteristic, flash and flight, and habitat. measured by visual estimation, measuring tape Using photography and videography to assist counting or a Leica DISTO DXT Laser Distance meter. The fireflies is introduced. Current limitations and further observer stand along the margins of the quadrat actions are proposed. to counts the number of fireflies displaying light ; or stand at the centre of the quadrat and count the Key words: Fireflies, Lampyridae, Rhagophthalmidae, number of fireflies displaying light in a 360 degree Hong Kong, local restrictedness, accessing population perspective – point count. c. Transect count. This was usually done by walking INTRODUCTION slowly along a road, a trail or a path; fireflies occurring on both sides of the path were counted. -

Coleoptera: Cucujoidea) Matthew Immelg Louisiana State University and Agricultural and Mechanical College, [email protected]
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2011 Revision and Reclassification of the Genera of Phalacridae (Coleoptera: Cucujoidea) Matthew immelG Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Entomology Commons Recommended Citation Gimmel, Matthew, "Revision and Reclassification of the Genera of Phalacridae (Coleoptera: Cucujoidea)" (2011). LSU Doctoral Dissertations. 2857. https://digitalcommons.lsu.edu/gradschool_dissertations/2857 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. REVISION AND RECLASSIFICATION OF THE GENERA OF PHALACRIDAE (COLEOPTERA: CUCUJOIDEA) A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Matthew Gimmel B.S., Oklahoma State University, 2005 August 2011 ACKNOWLEDGMENTS I would like to thank the following individuals for accommodating and assisting me at their respective institutions: Roger Booth and Max Barclay (BMNH), Azadeh Taghavian (MNHN), Phil Perkins (MCZ), Warren Steiner (USNM), Joe McHugh (UGCA), Ed Riley (TAMU), Mike Thomas and Paul Skelley (FSCA), Mike Ivie (MTEC/MAIC/WIBF), Richard Brown and Terry Schiefer (MEM), Andy Cline (CDFA), Fran Keller and Steve Heydon (UCDC), Cheryl Barr (EMEC), Norm Penny and Jere Schweikert (CAS), Mike Caterino (SBMN), Michael Wall (SDMC), Don Arnold (OSEC), Zack Falin (SEMC), Arwin Provonsha (PURC), Cate Lemann and Adam Slipinski (ANIC), and Harold Labrique (MHNL). -

A03v24n3.Pdf
Revista peruana de biología 24(3): 243 - 248 (2017) ISSN-L 1561-0837 A New Species of DODECACIUS (Coleoptera: Elateridae) from Madre de Dios, Peru doi: http://dx.doi.org/10.15381/rpb.v24i3.13903 Facultad de Ciencias Biológicas UNMSM TRABAJOS ORIGINALES A New Species of Dodecacius Schwarz (Coleoptera: Elateridae) from Madre de Dios, Peru Una nueva especie de Dodecacius Schwarz (Coleoptera: Elateridae) de Madre de Dios, Perú Paul J. Johnson Insect Biodiversity Lab, Box 2207A, South Dakota State University, Brookings, South Dakota 57007, U.S.A. Email: [email protected] Abstract Dodecacius Schwarz is reviewed, it includes two species known only from the eastern lower slopes of the Andes and adjacent Amazonia in southeastern Peru. Dodecacius paititi new species is described. Dodecacius testaceus Schwarz is treated as a new synonym of D. nigricollis Schwarz. Keywords: taxonomy; endemic; Andes; Amazonia; species discovery. Resumen El género Dodecacius Schwarz es revisado, incluye dos especies conocidas solamente de las laderas orientales bajas de los Andes y la Amazonia adyacente en el sureste de Perú. Se describe la nueva especie Dodecacius paititi y Dodecacius testaceus Schwarz es considerado como un nuevo sinónimo de D. nigricollis Schwarz. Palabras clave: taxonomía; endemismo; Andes; Amazonia; descubrimiento de especies. Publicación registrada en Zoobank/ZooBank article registered: urn:lsid:zoobank.org:pub:CF42CC9C-F496-4B4F-9C1A-FBB413A43E02 Acto nomenclatural/nomenclatural act: urn:lsid:zoobank.org:act:84A545F1-FAF8-42C1-83DA-C9D90CA0CA39 Citation: Johnson P.J. 2017. A New Species of Dodecacius Schwarz (Coleoptera: Elateridae) from Madre de Dios, Peru. Revista peruana de biología 24(3): 243 - 248 (octubre 2017). -

The Evolution and Genomic Basis of Beetle Diversity
The evolution and genomic basis of beetle diversity Duane D. McKennaa,b,1,2, Seunggwan Shina,b,2, Dirk Ahrensc, Michael Balked, Cristian Beza-Bezaa,b, Dave J. Clarkea,b, Alexander Donathe, Hermes E. Escalonae,f,g, Frank Friedrichh, Harald Letschi, Shanlin Liuj, David Maddisonk, Christoph Mayere, Bernhard Misofe, Peyton J. Murina, Oliver Niehuisg, Ralph S. Petersc, Lars Podsiadlowskie, l m l,n o f l Hans Pohl , Erin D. Scully , Evgeny V. Yan , Xin Zhou , Adam Slipinski , and Rolf G. Beutel aDepartment of Biological Sciences, University of Memphis, Memphis, TN 38152; bCenter for Biodiversity Research, University of Memphis, Memphis, TN 38152; cCenter for Taxonomy and Evolutionary Research, Arthropoda Department, Zoologisches Forschungsmuseum Alexander Koenig, 53113 Bonn, Germany; dBavarian State Collection of Zoology, Bavarian Natural History Collections, 81247 Munich, Germany; eCenter for Molecular Biodiversity Research, Zoological Research Museum Alexander Koenig, 53113 Bonn, Germany; fAustralian National Insect Collection, Commonwealth Scientific and Industrial Research Organisation, Canberra, ACT 2601, Australia; gDepartment of Evolutionary Biology and Ecology, Institute for Biology I (Zoology), University of Freiburg, 79104 Freiburg, Germany; hInstitute of Zoology, University of Hamburg, D-20146 Hamburg, Germany; iDepartment of Botany and Biodiversity Research, University of Wien, Wien 1030, Austria; jChina National GeneBank, BGI-Shenzhen, 518083 Guangdong, People’s Republic of China; kDepartment of Integrative Biology, Oregon State -

The Earliest Record of Fossil Solid-Wood-Borer Larvae—Immature Beetles in 99 Million-Year-Old Myanmar Amber
Palaeoentomology 004 (4): 390–404 ISSN 2624-2826 (print edition) https://www.mapress.com/j/pe/ PALAEOENTOMOLOGY Copyright © 2021 Magnolia Press Article ISSN 2624-2834 (online edition) PE https://doi.org/10.11646/palaeoentomology.4.4.14 http://zoobank.org/urn:lsid:zoobank.org:pub:9F96DA9A-E2F3-466A-A623-0D1D6689D345 The earliest record of fossil solid-wood-borer larvae—immature beetles in 99 million-year-old Myanmar amber CAROLIN HAUG1, 2, *, GIDEON T. HAUG1, ANA ZIPPEL1, SERITA VAN DER WAL1 & JOACHIM T. HAUG1, 2 1Ludwig-Maximilians-Universität München, Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany 2GeoBio-Center at LMU, Richard-Wagner-Str. 10, 80333 München, Germany �[email protected]; https://orcid.org/0000-0001-9208-4229 �[email protected]; https://orcid.org/0000-0002-6963-5982 �[email protected]; https://orcid.org/0000-0002-6509-4445 �[email protected] https://orcid.org/0000-0002-7426-8777 �[email protected]; https://orcid.org/0000-0001-8254-8472 *Corresponding author Abstract different plants, including agriculturally important ones (e.g., Potts et al., 2010; Powney et al., 2019). On the Interactions between animals and plants represent an other hand, many representatives exploit different parts of important driver of evolution. Especially the group Insecta plants, often causing severe damage up to the loss of entire has an enormous impact on plants, e.g., by consuming them. crops (e.g., Metcalf, 1996; Evans et al., 2007; Oliveira et Among beetles, the larvae of different groups (Buprestidae, Cerambycidae, partly Eucnemidae) bore into wood and are al., 2014). -

Crop Ecology, Cultivation and Uses of Cactus Pear
CROP ECOLOGY, CULTIVATION AND USES OF CACTUS PEAR Advance draft prepared for the IX INTERNATIONAL CONGRESS ON CACTUS PEAR AND COCHINEAL CAM crops for a hotter and drier world Coquimbo, Chile, 26-30 March 2017 CROP ECOLOGY, CULTIVATION AND USES OF CACTUS PEAR Editorial team Prof. Paolo Inglese, Università degli Studi di Palermo, Italy; General Coordinator Of the Cactusnet Dr. Candelario Mondragon, INIFAP, Mexico Dr. Ali Nefzaoui, ICARDA, Tunisia Prof. Carmen Sáenz, Universidad de Chile, Chile Coordination team Makiko Taguchi, FAO Harinder Makkar, FAO Mounir Louhaichi, ICARDA Editorial support Ruth Duffy Book design and layout Davide Moretti, Art&Design − Rome Published by the Food and Agriculture Organization of the United Nations and the International Center for Agricultural Research in the Dry Areas Rome, 2017 The designations employed and the FAO encourages the use, reproduction and presentation of material in this information dissemination of material in this information product do not imply the expression of any product. Except where otherwise indicated, opinion whatsoever on the part of the Food material may be copied, downloaded and Agriculture Organization of the United and printed for private study, research Nations (FAO), or of the International Center and teaching purposes, or for use in non- for Agricultural Research in the Dry Areas commercial products or services, provided (ICARDA) concerning the legal or development that appropriate acknowledgement of FAO status of any country, territory, city or area as the source and copyright holder is given or of its authorities, or concerning the and that FAO’s endorsement of users’ views, delimitation of its frontiers or boundaries. -

Hidden Diversity in the Brazilian Atlantic Rainforest
www.nature.com/scientificreports Corrected: Author Correction OPEN Hidden diversity in the Brazilian Atlantic rainforest: the discovery of Jurasaidae, a new beetle family (Coleoptera, Elateroidea) with neotenic females Simone Policena Rosa1, Cleide Costa2, Katja Kramp3 & Robin Kundrata4* Beetles are the most species-rich animal radiation and are among the historically most intensively studied insect groups. Consequently, the vast majority of their higher-level taxa had already been described about a century ago. In the 21st century, thus far, only three beetle families have been described de novo based on newly collected material. Here, we report the discovery of a completely new lineage of soft-bodied neotenic beetles from the Brazilian Atlantic rainforest, which is one of the most diverse and also most endangered biomes on the planet. We identifed three species in two genera, which difer in morphology of all life stages and exhibit diferent degrees of neoteny in females. We provide a formal description of this lineage for which we propose the new family Jurasaidae. Molecular phylogeny recovered Jurasaidae within the basal grade in Elateroidea, sister to the well-sclerotized rare click beetles, Cerophytidae. This placement is supported by several larval characters including the modifed mouthparts. The discovery of a new beetle family, which is due to the limited dispersal capability and cryptic lifestyle of its wingless females bound to long-term stable habitats, highlights the importance of the Brazilian Atlantic rainforest as a top priority area for nature conservation. Coleoptera (beetles) is by far the largest insect order by number of described species. Approximately 400,000 species have been described, and many new ones are still frequently being discovered even in regions with histor- ically high collecting activity1.