A Molecular Phylogeny of Lampyridae with Insight Into Visual and Bioluminescent Evolution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topic Paper Chilterns Beechwoods
. O O o . 0 O . 0 . O Shoping growth in Docorum Appendices for Topic Paper for the Chilterns Beechwoods SAC A summary/overview of available evidence BOROUGH Dacorum Local Plan (2020-2038) Emerging Strategy for Growth COUNCIL November 2020 Appendices Natural England reports 5 Chilterns Beechwoods Special Area of Conservation 6 Appendix 1: Citation for Chilterns Beechwoods Special Area of Conservation (SAC) 7 Appendix 2: Chilterns Beechwoods SAC Features Matrix 9 Appendix 3: European Site Conservation Objectives for Chilterns Beechwoods Special Area of Conservation Site Code: UK0012724 11 Appendix 4: Site Improvement Plan for Chilterns Beechwoods SAC, 2015 13 Ashridge Commons and Woods SSSI 27 Appendix 5: Ashridge Commons and Woods SSSI citation 28 Appendix 6: Condition summary from Natural England’s website for Ashridge Commons and Woods SSSI 31 Appendix 7: Condition Assessment from Natural England’s website for Ashridge Commons and Woods SSSI 33 Appendix 8: Operations likely to damage the special interest features at Ashridge Commons and Woods, SSSI, Hertfordshire/Buckinghamshire 38 Appendix 9: Views About Management: A statement of English Nature’s views about the management of Ashridge Commons and Woods Site of Special Scientific Interest (SSSI), 2003 40 Tring Woodlands SSSI 44 Appendix 10: Tring Woodlands SSSI citation 45 Appendix 11: Condition summary from Natural England’s website for Tring Woodlands SSSI 48 Appendix 12: Condition Assessment from Natural England’s website for Tring Woodlands SSSI 51 Appendix 13: Operations likely to damage the special interest features at Tring Woodlands SSSI 53 Appendix 14: Views About Management: A statement of English Nature’s views about the management of Tring Woodlands Site of Special Scientific Interest (SSSI), 2003. -

A Synopsis of Aquatic Fireflies with Description of a New Species (Coleoptera) 539-562 © Wiener Coleopterologenverein, Zool.-Bot
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Water Beetles of China Jahr/Year: 2003 Band/Volume: 3 Autor(en)/Author(s): Jeng Ming-Luen, Lai Jennifer, Yang Ping-Shih Artikel/Article: Lampyridae: A synopsis of aquatic fireflies with description of a new species (Coleoptera) 539-562 © Wiener Coleopterologenverein, Zool.-Bot. Ges. Österreich, Austria; download unter www.biologiezentrum.at JÄcil & Jl (eels.): Water Hectics of China Vol.111 539 - 562 Wien, April 2003 LAMPYRIDAE: A synopsis of aquatic fireflies with description of a new species (Coleoptera) M.-L. JENG, J. LAI & P.-S. YANG Abstract A synopsis of the Lampyridae (Coleoptera) hitherto reported to be aquatic is given. The authors could confirm aquatic larval stages for five out of the fifteen reported cases: Luciola cruciata MOTSCHULSKY (Japan), L. ficta OLIVIER (China, incl. Taiwan), L. latcralis MOTSCHULSKY (Japan, Korea, China and Russia), L. owadai SATO & KlMURA (Japan) and L. substriata Gorham (= L. fonnosana PIC syn.n.) (Taiwan, Myanmar and India). A sixth species, L. hyclrophila sp.n. (Taiwan), is described. The larvae of all but L. substriata have lateral tracheal gills on abdominal segments 1-8; L. substriata has a metapneustic larval stage with a pair of functional spiracles on the eighth abdominal segment. It is suggested that the aquatic habits in Luciola LAPORTE have evolved at least twice. The species with facultatively aquatic larvae are summarized also. A lectotype is designated for L.ficta. Key words: Coleoptera, Lampyridae, Luciola, aquatic, new species. Introduction Lampyridae, or fireflies, belong to the superfamily Cantharoidea (sensu CROWSON 1972) or Elatcroidea (sensu LAWRENCE & NEWTON 1995). -
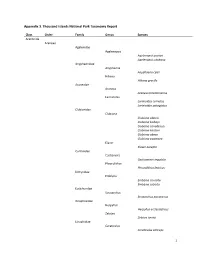
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Newsletter of the Biological Survey of Canada
Newsletter of the Biological Survey of Canada Vol. 40(1) Summer 2021 The Newsletter of the BSC is published twice a year by the In this issue Biological Survey of Canada, an incorporated not-for-profit From the editor’s desk............2 group devoted to promoting biodiversity science in Canada. Membership..........................3 President’s report...................4 BSC Facebook & Twitter...........5 Reminder: 2021 AGM Contributing to the BSC The Annual General Meeting will be held on June 23, 2021 Newsletter............................5 Reminder: 2021 AGM..............6 Request for specimens: ........6 Feature Articles: Student Corner 1. City Nature Challenge Bioblitz Shawn Abraham: New Student 2021-The view from 53.5 °N, Liaison for the BSC..........................7 by Greg Pohl......................14 Mayflies (mainlyHexagenia sp., Ephemeroptera: Ephemeridae): an 2. Arthropod Survey at Fort Ellice, MB important food source for adult by Robert E. Wrigley & colleagues walleye in NW Ontario lakes, by A. ................................................18 Ricker-Held & D.Beresford................8 Project Updates New book on Staphylinids published Student Corner by J. Klimaszewski & colleagues......11 New Student Liaison: Assessment of Chironomidae (Dip- Shawn Abraham .............................7 tera) of Far Northern Ontario by A. Namayandeh & D. Beresford.......11 Mayflies (mainlyHexagenia sp., Ephemerop- New Project tera: Ephemeridae): an important food source Help GloWorm document the distribu- for adult walleye in NW Ontario lakes, tion & status of native earthworms in by A. Ricker-Held & D.Beresford................8 Canada, by H.Proctor & colleagues...12 Feature Articles 1. City Nature Challenge Bioblitz Tales from the Field: Take me to the River, by Todd Lawton ............................26 2021-The view from 53.5 °N, by Greg Pohl..............................14 2. -

Methodologies for Monitoring Fireflies in Hong Kong
40 Methodologies for monitoring fireflies Methodologies for monitoring fireflies in Hong Kong Yiu Vor 31E, Tin Sam Tsuen, Kam Sheung Road, Yuen Long, N.T., Hong Kong. Email: [email protected] ABSTRACT record fireflies qualitatively and quantitatively, including: In total 241 field visits to 47 different sites in Hong Kong a. Malaise traps. Ten traps were set for a general were conducted specifically for firefly survey, from 2009 insects study in 2014 and small quantity of fireflies to 2020. Various methods were used to record fireflies were collected; qualitatively and quantitatively. Local restrictedness of 29 species of Hong Kong fireflies are listed. Methods b. Quadrat count and point count. Used in high for accessing the population of different firefly species visibility areas with concentration of flying fireflies are discussed and recommended according to their displaying light at night. Area of the quadrats was distribution characteristic, flash and flight, and habitat. measured by visual estimation, measuring tape Using photography and videography to assist counting or a Leica DISTO DXT Laser Distance meter. The fireflies is introduced. Current limitations and further observer stand along the margins of the quadrat actions are proposed. to counts the number of fireflies displaying light ; or stand at the centre of the quadrat and count the Key words: Fireflies, Lampyridae, Rhagophthalmidae, number of fireflies displaying light in a 360 degree Hong Kong, local restrictedness, accessing population perspective – point count. c. Transect count. This was usually done by walking INTRODUCTION slowly along a road, a trail or a path; fireflies occurring on both sides of the path were counted. -

By Joseph Michael Cicero a Thesis Submitted to the F
Evolution of the glow-signal system in Microphotus (Coleoptera, Lampyridae) Item Type text; Thesis-Reproduction (electronic) Authors Cicero, Joseph Michael Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 01/10/2021 11:55:43 Link to Item http://hdl.handle.net/10150/557669 EVOLUTION OF THE GLOW-SIGNAL SYSTEM IN MICROPHOTUS (COLEOPTERA, LAMPYRIDAE) by Joseph Michael Cicero A Thesis Submitted to the Faculty of the DEPARTMENT OF ENTOMOLOGY In Partial Fulfillment of the Requirements For the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 19 8 1 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfillment of requirements for an advanced degree at The University of Arizona and is deposited in the University Library to be made available to borrowers under rules of the Library. Brief quotations from this thesis are allowable without special permission, provided that accurate acknowledgement of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in his judgment the proposed use of the material is in the interests of scholarship. In all other instances, however, permission must be obtained from the author. APPROVAL BY THESIS DIRECTOR This thesis has been approved.on the date shown below: so, z//y F(LbYD G. -

Nuptial Food Gifts Influence Female Egg Production in the Scorpionfly
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Publications at Bielefeld University Ecological Entomology (2007), 32, 327–332 DOI: 10.1111/j.1365-2311.2006.00835.x SHORT COMMUNICATION Nuptial food gifts infl uence female egg production in the scorpionfl y Panorpa cognata LEIF ENGQVIST Department of Evolutionary Biology and Ecology, University of Bonn, Germany Abstract . 1. Before copulation, male Panorpa cognata scorpionflies offer females a salivary secretion, which is consumed by the female during copulation. It has previously been demonstrated that this nuptial food gift functions as mating effort by increasing male attractiveness and by increasing ejaculate transfer during copulation. 2. In this study, the effect of saliva consumption on female reproductive output was investigated, and thus the possibility that nuptial food gifts also serve as paternal investment. The experimental design enabled the effect of nuptial gift consumption to be disentangled from other possible effects of multiple mating or increased copula duration. 3. The results showed that saliva consumption increases female egg production by on average 8% (4.5 eggs) per consumed salivary mass, whereas mean egg weight was not influenced. 4. These results have important implications for the evolution and maintenance of both male nuptial gifts and female polyandry in this and other species. Key words . Female fecundity , mating effort , Mecoptera , nuptial food gifts , paternal investment , sexual selection . Introduction number or size of nuptial gifts on the female fitness traits stud- ied (e.g. Gwynne et al. , 1984; Jones et al. , 1986; Svärd & The traditional view that female fitness should be maximized Wiklund, 1988; Wedell & Arak, 1989; Will & Sakaluk, 1994; with only one or a few matings ( Bateman, 1948 ) is often chal- Ward & Landolt, 1995; Vahed & Gilbert, 1997; Cook, 1999; lenged by the observation of female multiple matings in many Maxwell, 2000; Ryne et al. -

Molecular Systematics of the Firefly Genus Luciola
animals Article Molecular Systematics of the Firefly Genus Luciola (Coleoptera: Lampyridae: Luciolinae) with the Description of a New Species from Singapore Wan F. A. Jusoh 1,* , Lesley Ballantyne 2, Su Hooi Chan 3, Tuan Wah Wong 4, Darren Yeo 5, B. Nada 6 and Kin Onn Chan 1,* 1 Lee Kong Chian Natural History Museum, National University of Singapore, Singapore 117377, Singapore 2 School of Agricultural and Wine Sciences, Charles Sturt University, Wagga Wagga 2678, Australia; [email protected] 3 Central Nature Reserve, National Parks Board, Singapore 573858, Singapore; [email protected] 4 National Parks Board HQ (Raffles Building), Singapore Botanic Gardens, Singapore 259569, Singapore; [email protected] 5 Department of Biological Sciences, National University of Singapore, Singapore 117543, Singapore; [email protected] 6 Forest Biodiversity Division, Forest Research Institute Malaysia, Kepong 52109, Malaysia; [email protected] * Correspondence: [email protected] (W.F.A.J.); [email protected] (K.O.C.) Simple Summary: Fireflies have a scattered distribution in Singapore but are not as uncommon as many would generally assume. A nationwide survey of fireflies in 2009 across Singapore documented 11 species, including “Luciola sp. 2”, which is particularly noteworthy because the specimens were collected from a freshwater swamp forest in the central catchment area of Singapore and did not fit Citation: Jusoh, W.F.A.; Ballantyne, the descriptions of any known Luciola species. Ten years later, we revisited the same locality to collect L.; Chan, S.H.; Wong, T.W.; Yeo, D.; new specimens and genetic material of Luciola sp. 2. Subsequently, the mitochondrial genome of that Nada, B.; Chan, K.O. -

A Global Perspective on Firefly Extinction Threats
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/339213788 A Global Perspective on Firefly Extinction Threats Article in BioScience · February 2020 DOI: 10.1093/biosci/biz157 CITATION READS 1 231 6 authors, including: Sara M Lewis Avalon Celeste Stevahn Owens Tufts University Tufts University 112 PUBLICATIONS 4,372 CITATIONS 10 PUBLICATIONS 48 CITATIONS SEE PROFILE SEE PROFILE Candace E. Fallon Sarina Jepsen The Xerces Society for Invertebrate Conservation The Xerces Society for Invertebrate Conservation 7 PUBLICATIONS 20 CITATIONS 36 PUBLICATIONS 283 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Usage of necrophagous beetles (Coleoptera) in forensic entomology: determination and developmental models View project Utilizing beetle larvae of family Silphidae in forensic practice View project All content following this page was uploaded by Sara M Lewis on 12 February 2020. The user has requested enhancement of the downloaded file. Forum A Global Perspective on Firefly Extinction Threats SARA M. LEWIS , CHOONG HAY WONG, AVALON C.S. OWENS , CANDACE FALLON, SARINA JEPSEN, ANCHANA THANCHAROEN, CHIAHSIUNG WU, RAPHAEL DE COCK, MARTIN NOVÁK, TANIA LÓPEZ-PALAFOX, VERONICA KHOO, AND J. MICHAEL REED Insect declines and their drivers have attracted considerable recent attention. Fireflies and glowworms are iconic insects whose conspicuous bioluminescent courtship displays carry unique cultural significance, giving them economic value as ecotourist attractions. Despite evidence of declines, a comprehensive review of the conservation status and threats facing the approximately 2000 firefly species worldwide is lacking. We conducted a survey of experts from diverse geographic regions to identify the most prominent perceived threats to firefly population and species persistence. -

Streams in Kume-Jima
Streams rich in biodiversity, with endemic species including Kikuzato’s Stream Snake and Kumejima Firefl y Streams in Kume-jima Permanent and Seasonal Streams Geographical Coordinates: 26°22’N, 126°46’E / Altitude: 120-280m / Area: 255ha / Major type of wetland: Permanent and seasonal streams / Designation: Natural Habitat Conservation Area under the Law for the Conservation of Endangered Species of Wild Fauna and Flora / Municipalities involved: Kumejima Town, Okinawa Prefecture / Ramsar designation: October 2008 / Ramsar Criteria: 2 Kumejima Firefl ies fl ashing in unison Upper reach of Shirase River Middle reaches of Shirase River View of the designated area (right hand side) from Uegusukudake Kumejima General Overview: Minami Kume-jima is an island located approxi- Freshwater Crab mately 100km west of the main island of Okinawa. The island has an area of ap- proximately 5900ha with a shoreline that stretches approximately 48km. In the northern area of the island, the ridge of Kikuzato’s Stream hills including O-take (230m) and Uegu- Snake suku-dake (309m) runs from west to east in an arching line. In the southeast, a gen- tly sloping terrain extends with the Uraji There are two facilities, Kumejima Na- [Other Rare Species] Reptile: Yamashina’s river and Shirase river running through it. ture & Culture Center and Kumejima Firefl y Ground Gecko Goniurosaurus kuroiwae Steep slopes face the sea in the north. Museum, for conservation management yamashinae, Kumejimahai Sinomicrurus With a mean annual temperature of 22.4 and public awareness. japonicus takarai / Amphibian: Ryukyu degrees C and an annual precipitation of [Kikuzato’s Stream Snake Opisthotropis Brown Frog Rana okinavana / Birds: Japa- approximately 2200mm, the hills in such kikuzatoi] This Snake is an endemic snake nese Wood Pigeon Columba janthina / sub-tropical climate are covered with to the streams in Kume-jima island with Crustacea: Kumejima Minami Freshwa- dense forest dominated by Castanopsis a body length of 60cm and a dark brown ter Crab Candidiopotamon kumejimense, sieboldii ssp. -

Insects of Larose Forest (Excluding Lepidoptera and Odonates)
Insects of Larose Forest (Excluding Lepidoptera and Odonates) • Non-native species indicated by an asterisk* • Species in red are new for the region EPHEMEROPTERA Mayflies Baetidae Small Minnow Mayflies Baetidae sp. Small minnow mayfly Caenidae Small Squaregills Caenidae sp. Small squaregill Ephemerellidae Spiny Crawlers Ephemerellidae sp. Spiny crawler Heptageniiidae Flatheaded Mayflies Heptageniidae sp. Flatheaded mayfly Leptophlebiidae Pronggills Leptophlebiidae sp. Pronggill PLECOPTERA Stoneflies Perlodidae Perlodid Stoneflies Perlodid sp. Perlodid stonefly ORTHOPTERA Grasshoppers, Crickets and Katydids Gryllidae Crickets Gryllus pennsylvanicus Field cricket Oecanthus sp. Tree cricket Tettigoniidae Katydids Amblycorypha oblongifolia Angular-winged katydid Conocephalus nigropleurum Black-sided meadow katydid Microcentrum sp. Leaf katydid Scudderia sp. Bush katydid HEMIPTERA True Bugs Acanthosomatidae Parent Bugs Elasmostethus cruciatus Red-crossed stink bug Elasmucha lateralis Parent bug Alydidae Broad-headed Bugs Alydus sp. Broad-headed bug Protenor sp. Broad-headed bug Aphididae Aphids Aphis nerii Oleander aphid* Paraprociphilus tesselatus Woolly alder aphid Cicadidae Cicadas Tibicen sp. Cicada Cicadellidae Leafhoppers Cicadellidae sp. Leafhopper Coelidia olitoria Leafhopper Cuernia striata Leahopper Draeculacephala zeae Leafhopper Graphocephala coccinea Leafhopper Idiodonus kelmcottii Leafhopper Neokolla hieroglyphica Leafhopper 1 Penthimia americana Leafhopper Tylozygus bifidus Leafhopper Cercopidae Spittlebugs Aphrophora cribrata -

Orthopteran Communities in the Conifer-Broadleaved Woodland Zone of the Russian Far East
Eur. J. Entomol. 105: 673–680, 2008 http://www.eje.cz/scripts/viewabstract.php?abstract=1384 ISSN 1210-5759 (print), 1802-8829 (online) Orthopteran communities in the conifer-broadleaved woodland zone of the Russian Far East THOMAS FARTMANN, MARTIN BEHRENS and HOLGER LORITZ* University of Münster, Institute of Landscape Ecology, Department of Community Ecology, Robert-Koch-Str. 26, D-48149 Münster, Germany; e-mail: [email protected] Key words. Orthoptera, cricket, grasshopper, community ecology, disturbance, grassland, woodland zone, Lazovsky Reserve, Russian Far East, habitat heterogeneity, habitat specifity, Palaearctic Abstract. We investigate orthopteran communities in the natural landscape of the Russian Far East and compare the habitat require- ments of the species with those of the same or closely related species found in the largely agricultural landscape of central Europe. The study area is the 1,200 km2 Lazovsky State Nature Reserve (Primorsky region, southern Russian Far East) 200 km east of Vladi- vostok in the southern spurs of the Sikhote-Alin Mountains (134°E/43°N). The abundance of Orthoptera was recorded in August and September 2001 based on the number present in 20 randomly placed 1 m² quadrates per site. For each plot (i) the number of species of Orthoptera, (ii) absolute species abundance and (iii) fifteen environmental parameters characterising habitat structure and micro- climate were recorded. Canonical correspondence analysis (CCA) was used first to determine whether the Orthoptera occur in ecol- ogically coherent groups, and second, to assess their association with habitat characteristics. In addition, the number of species and individuals in natural and semi-natural habitats were compared using a t test.