Genetic Evidence That Celsr3 and Celsr2, Together with Fzd3, Regulate Forebrain Wiring in a Vangl-Independent Manner
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Transcriptomic Analysis of Native Versus Cultured Human and Mouse Dorsal Root Ganglia Focused on Pharmacological Targets Short
bioRxiv preprint doi: https://doi.org/10.1101/766865; this version posted September 12, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Transcriptomic analysis of native versus cultured human and mouse dorsal root ganglia focused on pharmacological targets Short title: Comparative transcriptomics of acutely dissected versus cultured DRGs Andi Wangzhou1, Lisa A. McIlvried2, Candler Paige1, Paulino Barragan-Iglesias1, Carolyn A. Guzman1, Gregory Dussor1, Pradipta R. Ray1,#, Robert W. Gereau IV2, # and Theodore J. Price1, # 1The University of Texas at Dallas, School of Behavioral and Brain Sciences and Center for Advanced Pain Studies, 800 W Campbell Rd. Richardson, TX, 75080, USA 2Washington University Pain Center and Department of Anesthesiology, Washington University School of Medicine # corresponding authors [email protected], [email protected] and [email protected] Funding: NIH grants T32DA007261 (LM); NS065926 and NS102161 (TJP); NS106953 and NS042595 (RWG). The authors declare no conflicts of interest Author Contributions Conceived of the Project: PRR, RWG IV and TJP Performed Experiments: AW, LAM, CP, PB-I Supervised Experiments: GD, RWG IV, TJP Analyzed Data: AW, LAM, CP, CAG, PRR Supervised Bioinformatics Analysis: PRR Drew Figures: AW, PRR Wrote and Edited Manuscript: AW, LAM, CP, GD, PRR, RWG IV, TJP All authors approved the final version of the manuscript. 1 bioRxiv preprint doi: https://doi.org/10.1101/766865; this version posted September 12, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Celsr1-3 Cadherins in PCP and Brain Development
CHAPTER SEVEN Celsr1–3 Cadherins in PCP and Brain Development Camille Boutin, André M. Goffinet1, Fadel Tissir1 Institute of Neuroscience, Developmental Neurobiology, Universite´ Catholique de Louvain, Brussels, Belgium 1Corresponding authors: Equal contribution. e-mail address: [email protected]; andre. [email protected] Contents 1. Celsr1–3 Expression Patterns 164 2. Celsr1: A Major Player in Vertebrate PCP 165 3. Celsr2 and 3 in Ciliogenesis 169 4. Celsr1–3 in Neuronal Migration 171 5. Celsr2 and Celsr3 in Brain Wiring 174 5.1 Motifs of Celsr important for their functions 176 References 179 Abstract Cadherin EGF LAG seven-pass G-type receptors 1, 2, and 3 (Celsr1–3) form a family of three atypical cadherins with multiple functions in epithelia and in the nervous system. During the past decade, evidence has accumulated for important and distinct roles of Celsr1–3 in planar cell polarity (PCP) and brain development and maintenance. Although the role of Celsr in PCP is conserved from flies to mammals, other functions may be more distantly related, with Celsr working only with one or a subset of the classical PCP partners. Here, we review the literature on Celsr in PCP and neural devel- opment, point to several remaining questions, and consider future challenges and possible research trends. Celsr1–3 genes encode atypical cadherins of more than 3000 amino acids ( Fig. 7.1). Their large ectodomain is composed of nine N-terminal cadherin repeats (typical cadherins have five repeats), six epidermal growth factor (EGF)-like domains, two laminin G repeats, one hormone receptor motif (HRM), and a G-protein-coupled receptor proteolytic site (GPS). -

Table 1A SIRT1 Differential Binding Gene List Down
Rp1 Rb1cc1 Pcmtd1 Mybl1 Sgk3 Cspp1 Arfgef1 Cpa6 Kcnb2 Stau2 Jph1 Paqr8 Kcnq5 Rims1 Smap1 Bai3 Prim2 Bag2 Zfp451 Dst Uggt1 4632411B12Rik Fam178b Tmem131 Inpp4a 2010300C02Rik Rev1 Aff3 Map4k4 Il1r1 Il1rl2 Tgfbrap1 Col3a1 Wdr75 Tmeff2 Hecw2 Boll Plcl1 Satb2 Aox4 Mpp4 Gm973 Carf Nbeal1 Pard3b Ino80d Adam23 Dytn Pikfyve Atic Fn1 Smarcal1 Tns1 Arpc2 Pnkd Ctdsp1 Usp37 Acsl3 Cul3 Dock10 Col4a4 Col4a3 Mff Wdr69 Pid1 Sp110 Sp140 Itm2c 2810459M11Rik Dis3l2 Chrng Gigyf2 Ugt1a7c Ugt1a6b Hjurp A730008H23Rik Trpm8 Kif1a D1Ertd622e Pam Cntnap5b Rnf152 Phlpp1 Clasp1 Gli2 Dpp10 Tmem163 Zranb3 Pfkfb2 Pigr Rbbp5 Sox13 Ppfia4 Rabif Kdm5b Ppp1r12b Lgr6 Pkp1 Kif21b Nr5a2 Dennd1b Trove2 Pdc Hmcn1 Ncf2 Nmnat2 Lamc2 Cacna1e Xpr1 Tdrd5 Fam20b Ralgps2 Sec16b Astn1 Pappa2 Tnr Klhl20 Dnm3 Bat2l2 4921528O07Rik Scyl3 Dpt Mpzl1 Creg1 Cd247 Gm4846 Lmx1a Pbx1 Ddr2 Cd244 Cadm3 Fmn2 Grem2 Rgs7 Fh1 Wdr64 Pld5 Cep170 Akt3 Pppde1 Smyd3 Parp1 Enah Capn8 Susd4 Mosc1 Rrp15 Gpatch2 Esrrg Ptpn14 Smyd2 Tmem206 Nek2 Traf5 Hhat Cdnf Fam107b Camk1d Cugbp2 Gata3 Itih5 Sfmbt2 Itga8 Pter Rsu1 Ptpla Slc39a12 Cacnb2 Nebl Pip4k2a Abi1 Tbpl2 Pnpla7 Kcnt1 Camsap1 Nacc2 Gpsm1 Sec16a Fam163b Vav2 Olfm1 Tsc1 Med27 Rapgef1 Pkn3 Zer1 Prrx2 Gpr107 Ass1 Nup214 Bat2l Dnm1 Ralgps1 Fam125b Mapkap1 Hc Ttll11 Dennd1a Olfml2a Scai Arhgap15 Gtdc1 Mbd5 Kif5c Lypd6b Lypd6 Fmnl2 Arl6ip6 Galnt13 Acvr1 Ccdc148 Dapl1 Tanc1 Ly75 Gcg Kcnh7 Cobll1 Scn3a Scn9a Scn7a Gm1322 Stk39 Abcb11 Slc25a12 Metapl1 Pdk1 Rapgef4 B230120H23Rik Gpr155 Wipf1 Pde11a Prkra Gm14461 Pde1a Calcrl Olfr1033 Mybpc3 F2 Arhgap1 Ambra1 Dgkz Creb3l1 -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners. -

G Protein‐Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: G protein-coupled receptors. British Journal of Pharmacology (2019) 176, S21–S141 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors Stephen PH Alexander1 , Arthur Christopoulos2 , Anthony P Davenport3 , Eamonn Kelly4, Alistair Mathie5 , John A Peters6 , Emma L Veale5 ,JaneFArmstrong7 , Elena Faccenda7 ,SimonDHarding7 ,AdamJPawson7 , Joanna L Sharman7 , Christopher Southan7 , Jamie A Davies7 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Monash Institute of Pharmaceutical Sciences and Department of Pharmacology, Monash University, Parkville, Victoria 3052, Australia 3Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK 4School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 5Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 6Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 7Centre for Discovery Brain Sciences, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. Although the Concise Guide represents approximately 400 pages, the material presented is substantially reduced compared to information and links presented on the website. -

A Second Trigeminal CGRP Receptor: Function and Expression of the AMY1 Receptor
A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Walker, Christopher S; Eftekhari, Sajedeh; Bower, Rebekah L; Wilderman, Andrea; Insel, Paul A; Edvinsson, Lars; Waldvogel, Henry J; Jamaluddin, Muhammad A; Russo, Andrew F; Hay, Debbie L Published in: Annals of Clinical and Translational Neurology DOI: 10.1002/acn3.197 2015 Link to publication Citation for published version (APA): Walker, C. S., Eftekhari, S., Bower, R. L., Wilderman, A., Insel, P. A., Edvinsson, L., Waldvogel, H. J., Jamaluddin, M. A., Russo, A. F., & Hay, D. L. (2015). A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Annals of Clinical and Translational Neurology, 2(6), 595-608. https://doi.org/10.1002/acn3.197 Total number of authors: 10 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

Dystroglycan Is a Scaffold for Extracellular Axon Guidance Decisions L Bailey Lindenmaier1, Nicolas Parmentier2, Caiying Guo3, Fadel Tissir2, Kevin M Wright1*
RESEARCH ARTICLE Dystroglycan is a scaffold for extracellular axon guidance decisions L Bailey Lindenmaier1, Nicolas Parmentier2, Caiying Guo3, Fadel Tissir2, Kevin M Wright1* 1Vollum Institute, Oregon Health & Science University, Portland, United States; 2Institiute of Neuroscience, Universite´ Catholique de Louvain, Brussels, Belgium; 3Janelia Research Campus, Howard Hughes Medical Institute, Ashburn, United States Abstract Axon guidance requires interactions between extracellular signaling molecules and transmembrane receptors, but how appropriate context-dependent decisions are coordinated outside the cell remains unclear. Here we show that the transmembrane glycoprotein Dystroglycan interacts with a changing set of environmental cues that regulate the trajectories of extending axons throughout the mammalian brain and spinal cord. Dystroglycan operates primarily as an extracellular scaffold during axon guidance, as it functions non-cell autonomously and does not require signaling through its intracellular domain. We identify the transmembrane receptor Celsr3/ Adgrc3 as a binding partner for Dystroglycan, and show that this interaction is critical for specific axon guidance events in vivo. These findings establish Dystroglycan as a multifunctional scaffold that coordinates extracellular matrix proteins, secreted cues, and transmembrane receptors to regulate axon guidance. DOI: https://doi.org/10.7554/eLife.42143.001 Introduction During neural circuit development, extending axons encounter distinct combinations of cues and *For correspondence: growth substrates that guide their trajectory. These cues can be attractive or repulsive, secreted [email protected] and/or anchored to cell membranes, and signal through cell surface receptors on the growth cones of axons (Kolodkin and Tessier-Lavigne, 2011). Receptors also recognize permissive and non-per- Competing interest: See missive growth substrates formed by the extracellular matrix (ECM), surrounding cells, and other page 21 axons (Raper and Mason, 2010). -

GPCR Expression Profiles Were Determined Using
Supplemental Figures and Tables for Tischner et al., 2017 Supplemental Figure 1: GPCR expression profiles were determined using the NanoString nCounter System in 250 ng of pooled cell RNA obtained from freshly isolated CD4 T cells from naïve lymph nodes (CD4ln), spinal cord infiltrating CD4 T cells at peak EAE disease (CD4sc), and primary lung endothelial cells (luEC). Supplemental Figure 2: Array design and quality controls. A, Sorted leukocytes or endothelial cells were subjected to single‐cell expression analysis and re‐evaluated based on the expression of various identity‐defining genes. B, Expression of identity‐defining and quality control genes after deletion of contaminating or reference gene‐negative cells. Expression data are calculated as 2(Limit of detection(LoD) Ct – sample Ct) ; LoD Ct was set to 24. Supplemental Figure 3: Overview over GPCR expression frequencies in different freshly isolated immune cell populations and spinal cord endothelial cells as determined by single cell RT‐PCR. Abbreviations: CD4ln‐Tcon/CD4ln‐Treg, conventional (con) and regulatory (reg) CD4 T cells from lymph nodes (CD4ln) of naïve mice; CD4dr/CD4sc, CD4 T cells from draining lymph nodes (dr) or spinal cord (sc) at peak EAE disease; CD4spn2D/ CD4spn2DTh1/ CD4spn2DTh17, splenic CD4 T cells from 2D2 T cell receptor transgenic mice before (2D) and after in vitro differentiation towards Th1 (2DTh1) or Th17 (2DTh17); MonoSpn, splenic monocytes; CD11b_sc, spinal cord infiltrating CD11b‐ positive cells; sc_microglia, Ccr2neg,Cx3cr1pos microglia from spinal cord at peak disease; sc_macrophages, CCr2pos;Cx3cr1lo/neg macrophages from spinal cord at peak disease; BMDM_M1/BMDM_M2, bone marrow‐derived macrophages differentiated towards M1 or M2; ECscN and ECscEAE, spinal cord endothelial cells from naïve mice (N) and at peak EAE disease (EAE); SMC, smooth muscle cells from various vessel types (included as positive control to ascertain primer functionality). -
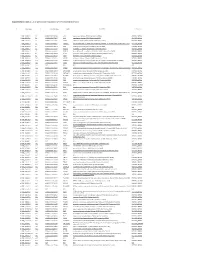
Supplemetary Table 2. List of Genes Down-Regulated in LPAR6 Knocked Down Cells
Supplemetary Table 2. List of genes down-regulated in LPAR6 knocked down cells g# initial alias c# converted alias name description namespace 1 NM_002317.5 1.1 ENSG00000113083 LOX lysyl oxidase [Source:HGNC Symbol;Acc:6664] REFSEQ_MRNA 2 NM_006183.4 2.1 ENSG00000133636 NTS neurotensin [Source:HGNC Symbol;Acc:8038] REFSEQ_MRNA 3 NM_005213.3 3.1 ENSG00000121552 CSTA cystatin A (stefin A) [Source:HGNC Symbol;Acc:2481] REFSEQ_MRNA 4 NM_007231.3 4.1 ENSG00000087916 SLC6A14 solute carrier family 6 (amino acid transporter), member 14 [Source:HGNC Symbol;Acc:11047] REFSEQ_MRNA 5 NM_001873.2 5.1 ENSG00000109472 CPE carboxypeptidase E [Source:HGNC Symbol;Acc:2303] REFSEQ_MRNA 6 NM_019856.1 6.1 ENSG00000101605 MYOM1 myomesin 1, 185kDa [Source:HGNC Symbol;Acc:7613] REFSEQ_MRNA 7 NM_032590.4 7.1 ENSG00000089094 KDM2B lysine (K)-specific demethylase 2B [Source:HGNC Symbol;Acc:13610] REFSEQ_MRNA 8 NM_001901.2 8.1 ENSG00000118523 CTGF connective tissue growth factor [Source:HGNC Symbol;Acc:2500] REFSEQ_MRNA 9 NM_007183.2 9.1 ENSG00000184363 PKP3 plakophilin 3 [Source:HGNC Symbol;Acc:9025] REFSEQ_MRNA 10 NM_182965.2 10.1 ENSG00000176170 SPHK1 sphingosine kinase 1 [Source:HGNC Symbol;Acc:11240] REFSEQ_MRNA 11 NM_152423.4 11.1 ENSG00000157502 MUM1L1 melanoma associated antigen (mutated) 1-like 1 [Source:HGNC Symbol;Acc:26583] REFSEQ_MRNA 12 NM_002923.3 12.1 ENSG00000116741 RGS2 regulator of G-protein signaling 2, 24kDa [Source:HGNC Symbol;Acc:9998] REFSEQ_MRNA 13 NR_003038.2 13.1 N/A N/A N/A N/A 14 NM_080862.1 14.1 ENSG00000175093 SPSB4 splA/ryanodine receptor -

Epigenetic Mechanisms Are Involved in the Oncogenic Properties of ZNF518B in Colorectal Cancer
Epigenetic mechanisms are involved in the oncogenic properties of ZNF518B in colorectal cancer Francisco Gimeno-Valiente, Ángela L. Riffo-Campos, Luis Torres, Noelia Tarazona, Valentina Gambardella, Andrés Cervantes, Gerardo López-Rodas, Luis Franco and Josefa Castillo SUPPLEMENTARY METHODS 1. Selection of genomic sequences for ChIP analysis To select the sequences for ChIP analysis in the five putative target genes, namely, PADI3, ZDHHC2, RGS4, EFNA5 and KAT2B, the genomic region corresponding to the gene was downloaded from Ensembl. Then, zoom was applied to see in detail the promoter, enhancers and regulatory sequences. The details for HCT116 cells were then recovered and the target sequences for factor binding examined. Obviously, there are not data for ZNF518B, but special attention was paid to the target sequences of other zinc-finger containing factors. Finally, the regions that may putatively bind ZNF518B were selected and primers defining amplicons spanning such sequences were searched out. Supplementary Figure S3 gives the location of the amplicons used in each gene. 2. Obtaining the raw data and generating the BAM files for in silico analysis of the effects of EHMT2 and EZH2 silencing The data of siEZH2 (SRR6384524), siG9a (SRR6384526) and siNon-target (SRR6384521) in HCT116 cell line, were downloaded from SRA (Bioproject PRJNA422822, https://www.ncbi. nlm.nih.gov/bioproject/), using SRA-tolkit (https://ncbi.github.io/sra-tools/). All data correspond to RNAseq single end. doBasics = TRUE doAll = FALSE $ fastq-dump -I --split-files SRR6384524 Data quality was checked using the software fastqc (https://www.bioinformatics.babraham. ac.uk /projects/fastqc/). The first low quality removing nucleotides were removed using FASTX- Toolkit (http://hannonlab.cshl.edu/fastxtoolkit/).