Compilation of Ground-Water-Quality Data in Pennsylvania
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

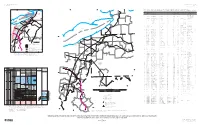
Lancaster County Geology
LancasterLancaster CountyCounty GeologyGeology gfgh µ OverOver TopographicTopographic ReliefRelief Om Miles Í897 0 10 hg Lebanon County Adamstown ¦¨§76 ! Berks County Oha ! Oo Denver ab322 Ephrata Csc Í501 ! Í72 TRh 272 TRn Oco Lititz Í ! Akron Elizabethtown Manheim ! Dauphin County! ! TRhc Cr TRs c Os Rn ! T Terre Hill TRg Trd 322 772 ab 10 Í 222 625 Í ab Í897 Í Í283 Ohm Í230 Oan Í241 East New Holland Oe 722 Petersburg Í Cbs ! Mount Joy ! ! Czc Cch Í23 Cm gga fl R 743 T Í Csb gg pg ggd Í441 Ck Cl 772 72 Í 23 Í ! Í Ca Marietta Ch Lancaster Mountville ! 340 ! Í Columbia 30 ! ! ba Í462 Í462 ab30 Í999 Millersville Ccc Strasburg ! ! Cv Í741 Í741 Í272 41 Í County Chester 896 222 Í gn Christiana ba mg ! oct Cah Í372 S u sq u e h a n n a R iv e r Y o rk C o u n ty gqm LEGEND COUNTY BOUNDARIES Í324 Source: Lancaster County GIS, Copyright (c) 2019 MAJOR ROADS This map to be used for reference or illustrative purposes only. This map FAULT is not a legally recorded plan, survey, or engineering schematic Quarryville and it is not intended to be used as such. For complete disclaimer see: RIVERS AND STREAMS ! http://www.co.lancaster.pa.us/gisdisclaimer DIKE wc ORDOVICIAN Í472 Oan, ANNVILLE FORMATION LIMESTONE TRIASSIC 372 Oco, COCALICO FORMATION DARK GRAY SHALE TRfl, LIMESTONE FANGLOMERATE Í TRg, GETTYSBURG FORMATION SHALE-SANDSTONE Oe, EPLER FORMATION LIMESTONE TRh, HAMMER CREEK FORMATION SANDSTONE-SHALE Oha, ANNVILLE, HERSHEY, AND MYERSTOWN FORMATION TRhc, HAMMER CREEK QUARTZ-CONGLOMERATE Ohm, HERSHEY AND MYERSTOWN FORMATION LIMESTONE 272 TRn, NEW -

USNMP-76 2806 1929.Pdf
NEW LOWER AND MIDDLE CAJ^IBRIAN CRUSTACEA By Charles E, Resser Associate Curator of Stratigraphic Paleontology INTRODUCTION Among the numerous Cambrian fossils that have been accumulating in the United States National Museum during the last 15 years, there are many undescribed species and some of the specimens are remarkable for the preservation of thin tests or of soft body parts. In order to stimulate further search for the rarer fossils, and par- ticularly for preservations of the softer parts of animals or of delicate plant tissues, it is planned to publish more or less related groups of these animals from time to time. Accordingly, in this paper I have assembled a group of species that centers mainly around the pre- viously described genus Tuzoia, but which also includes several unre- lated forms that were secured from the same localities as the others. This paper thus adds several new species preserving more than ordinarily thin tests of Crustacea and a few with the still softer fleshy parts. Some are from the well known Burgess shale that has already furnished so many interesting animals and plants, but other formations, some of which have not previously been known to yield such fossils, are also represented. This paper also contains information of interest aside from that naturally attaching to any description of the softer parts of such ancient animals, by presenting certain important stratigraphic facts in addition to further data regarding the geographic distribution and origin of the faunas to which these species belong. Acknowledgment.—In the preparation of this paper I was kindly assisted by Dr. -

Geological Investigations in Ohio
INFORMATION CIRCULAR NO. 21 GEOLOGICAL INVESTIGATIONS IN OHIO 1956 By Carolyn Farnsworth STATE OF OHIO C. William O'Neill, Governor DEPARTMENT OF NATURAL RESOURCES A. W. Marion, Director NATURAL RESOURCES COMMISSION Milton Ronsheim, Chairman John A. Slipher, Bryce Browning, Vice Chairman Secretary C. D. Blubaugh Dean L. L. Rummell Forrest G. Hall Dr. Myron T. Sturgeon A. W. Marion George Wenger DIVISION OF GEOLOGICAL SURVEY Ralph J. Bernhagen, Chief STATI OF OHIO DIPAlTMIMT 011 NATUlAL llSOUlCH DIVISION OF &EOLO&ICAL SURVEY INFORMATION CIRCULAR NO. 21 'GEOLOG·ICAL INVESTIGATIONS IN OHIO 1956 by CAROLYN FARNSWORTH COLUMBUS 1957 Blank Page CONTENTS Page Introduction 1 Project listing by author 2 Project listing by subject . 22 Economic geology 22 Aggregates . 22 Coal . • 22 Ground water 22 Iron .. 22 Oil and gas 22 Salt . 22 Sand and gravel 23 General .. 23 Geomorphology 23 Geophysics 23 Glacial geology 23 Mineralogy and petrology . 24 Clay .. 24 Coal . 24 Dolomite 24 Limestone. 24 Sandstone •• 24 Shale. 24 Till 25 Others 25 Paleontology. 25 Stratigraphy and sedimentation 26 Structural geology . 27 Miscellaneous . 27 Geographic distribution. 27 Statewide 27 Areal. \\ 28 County 29 Miscellaneous . 33 iii Blank Page I INTRODUCTION In September 1956, letters of inquiry and questionnaires were sent to all Ohio geologists on the mailing list of the Ohio Geological Survey, and to other persons who might be working on geological problems in Ohio. This publication has been compiled from the information contained on the returned forms. In most eases it is assumed that the projects listed herein will culminate in reports which will be available to the profession through scientific journals, government publications, or grad- uate school theses. -

Strophomenide and Orthotetide Silurian Brachiopods from the Baltic Region, with Particular Reference to Lithuanian Boreholes
Strophomenide and orthotetide Silurian brachiopods from the Baltic region, with particular reference to Lithuanian boreholes PETRAS MUSTEIKIS and L. ROBIN M. COCKS Musteikis, P. and Cocks, L.R.M. 2004. Strophomenide and orthotetide Silurian brachiopods from the Baltic region, with particular reference to Lithuanian boreholes. Acta Palaeontologica Polonica 49 (3): 455–482. Epeiric seas covered the east and west parts of the old craton of Baltica in the Silurian and brachiopods formed a major part of the benthic macrofauna throughout Silurian times (Llandovery to Pridoli). The orders Strophomenida and Orthotetida are conspicuous components of the brachiopod fauna, and thus the genera and species of the superfamilies Plec− tambonitoidea, Strophomenoidea, and Chilidiopsoidea, which occur in the Silurian of Baltica are reviewed and reidentified in turn, and their individual distributions are assessed within the numerous boreholes of the East Baltic, particularly Lithua− nia, and attributed to benthic assemblages. The commonest plectambonitoids are Eoplectodonta(Eoplectodonta)(6spe− cies), Leangella (2 species), and Jonesea (2 species); rarer forms include Aegiria and Eoplectodonta (Ygerodiscus), for which the new species E. (Y.) bella is erected from the Lithuanian Wenlock. Eight strophomenoid families occur; the rare Leptaenoideidae only in Gotland (Leptaenoidea, Liljevallia). Strophomenidae are represented by Katastrophomena (4 spe− cies), and Pentlandina (2 species); Bellimurina (Cyphomenoidea) is only from Oslo and Gotland. Rafinesquinidae include widespread Leptaena (at least 11 species) and Lepidoleptaena (2 species) with Scamnomena and Crassitestella known only from Gotland and Oslo. In the Amphistrophiidae Amphistrophia is widespread, and Eoamphistrophia, Eocymostrophia, and Mesodouvillina are rare. In the Leptostrophiidae Mesoleptostrophia, Brachyprion,andProtomegastrophia are com− mon, but Eomegastrophia, Eostropheodonta, Erinostrophia,andPalaeoleptostrophia are only recorded from the west in the Baltica Silurian. -

Geologic Resources Inventory Ancillary Map Information Document for Little River Canyon National Preserve
U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Directorate Geologic Resources Division Little River Canyon National Preserve GRI Ancillary Map Information Document Produced to accompany the Geologic Resources Inventory (GRI) Digital Geologic Data for Little River Canyon National Preserve liri_geology.pdf Version: 6/30/2020 I Little River Canyon National Preserve Geologic Resources Inventory Ancillary Map Information Document for Little River Canyon National Preserve Table of Contents Geologic Reso..u..r..c..e..s.. .I.n..v..e..n..t.o...r.y.. .M...a..p.. .D...o..c..u..m...e...n..t............................................................................ 1 About the NPS.. .G...e..o..l.o..g..i.c... .R..e..s..o..u...r.c..e..s.. .I.n..v..e..n...t.o..r.y.. .P...r.o..g...r.a..m............................................................... 3 GRI Digital Ma.p..s.. .a..n...d.. .S..o..u...r.c..e.. .M...a..p.. .C...i.t.a..t..i.o..n..s.................................................................................. 5 Index Ma..p........................................................................................................................................................................ 6 Map Unit List ................................................................................................................................... 7 Map Unit Desc..r.i.p..t.i.o...n..s...................................................................................................................... 9 Qal - Allu..v..iu..m... .a..n..d.. .l.o..w... .t.e..r.r.a..c..e.. .d..e..p..o..s..i.t.s. .(..Q..u..a..t.e..r..n..a..r.y.)..................................................................................................... 9 Tal - Fluv..i.a..l. .d..e..p..o..s.i.t.s.. .(.T..e..r..t.ia..r..y.)........................................................................................................................................ 9 PNpv - P.o..t.t.s..v..i.l.le.. .F...o..r.m...a..t.i.o..n. -

Historical Development and Problems Within the Pennsylvanian Nomenclature of Ohio.1
Historical Development and Problems Within the Pennsylvanian Nomenclature of Ohio.1 GLENN E. LARSEN, OHIO Department of Natural Resources, Division of Geological Survey, Fountain Sq., Bldg. B, Columbus, OH 43224 ABSTRACT. An analysis of the historical development of the Pennsylvanian stratigraphic nomenclature, as used in Ohio, has helped define and clarify problems inherent in Ohio's stratigraphic nomenclature. Resolution of such problems facilitates further development of a useful stratigraphy and philosophy for mapping. Investigations of Pennsylvanian-age rocks in Ohio began as early as 1819- From 1858 to 1893, investigations by Newberry, I. C. White, and Orton established the stratigraphic framework upon which the present-day nomenclature is based. During the 1950s, the cyclothem concept was used to classify and correlate Pennsylvanian lithologic units. This classification led to a proliferation of stratigraphic terms, as almost every lithologic type was named and designated as a member of a cyclothem. By the early 1960s, cyclothems were considered invalid as a lithostratigraphic classification. Currently, Pennsylvanian nomenclature of Ohio, as used by the Ohio Division of Geological Survey, consists of four groups containing 123 named beds, with no formal formations or members. In accordance with the 1983 North American Stratigraphic code, the Ohio Division of Geological Survey considers all nomenclature below group rank as informal. OHIO J. SCI. 91 (1): 69-76, 1991 INTRODUCTION DISCUSSION Understanding the historical development of Pennsyl- The Early 1800s vanian stratigraphy in Ohio is important to the Ohio The earliest known references to Pennsylvanian-age Division of Geological Survey (OGS). Such an under- rocks in Ohio are found in Atwater's (1819) report on standing of Pennsylvanian stratigraphy helps define Belmont County, and an article by Granger (1821) on plant stratigraphic nomenclatural problems in order to make fossils collected near Zanesville, Muskingum County. -

Gettysburg National Military Park & Eisenhower National Historic Site
National Park Service U.S. Department of the Interior Natural Resource Program Center Gettysburg National Military Park & Eisenhower National Historic Site Geologic Resources Inventory Report Natural Resource Report NPS/NRPC/GRD/NRR—2009/083 THIS PAGE: North Carolina State Monument (NPS Photo) ON THE COVER: Gettysburg NMP, looking toward Cemetery Ridge Cover photo by Bill Dowling, courtesy of the Gettysburg Foundation Gettysburg National Military Park and Eisenhower National Historic Site Geologic Resources Inventory Report Natural Resource Report NPS/NRPC/GRD/NRR—2009/083 Geologic Resources Division Natural Resource Program Center P.O. Box 25287 Denver, Colorado 80225 March 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Denver, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientific community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. Natural Resource Reports are the designated medium for disseminating high priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. Examples of the diverse array of reports published in this series include vital signs monitoring plans; "how to" resource management papers; proceedings of resource management workshops or conferences; annual reports of resource programs or divisions of the Natural Resource Program Center; resource action plans; fact sheets; and regularly-published newsletters. -

Summerfield and Woodsfield Quadrangles, Ohio
DEPARTMENT OF THE INTERIOR ALBERT B. FALL, Secretary UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, Director Bulletin 720 ECONOMIC GEOLOGY OF THE SUMMERFIELD AND WOODSFIELD QUADRANGLES, OHIO WITH DESCRIPTIONS OF COAL AND OTHER MINERAL RESOURCES EXCEPT OIL AND GAS BY D. DALE CONDIT WASHINGTON GOVERNMENT PRINTING OFFICE 1923 ADDITIONAL COPIES OF THIS PUBLICATION MAY BE PROCURED FROM THE SUPERINTENDENT OF DOCUMENTS GOVERNMENT PRINTING OFFICE WASHINGTON, D. C. AT 30 CENTS PER COPY CONTENTS. Bibliography............................................................. 6 Introduction. ^........................................................... 7 i Abstract of report.............................................:....... 7 Field and office work................................................. 7 Acknowledgments.................................................... 8 Geography .................../...................................... ..... 8 Location............................................................ 8 Topographic features................................................... 9 Drainage........................................................ 9 Relief and land forms...............:........... '. ................. 10 Agricultural and commercial conditions................................ 11 Transportation facilities........................................... 11 Railroads.................................................... 11 New railroad routes........................................... 11 Highways................................................... -

Figure 3A. Major Geologic Formations in West Virginia. Allegheney And
82° 81° 80° 79° 78° EXPLANATION West Virginia county boundaries A West Virginia Geology by map unit Quaternary Modern Reservoirs Qal Alluvium Permian or Pennsylvanian Period LTP d Dunkard Group LTP c Conemaugh Group LTP m Monongahela Group 0 25 50 MILES LTP a Allegheny Formation PENNSYLVANIA LTP pv Pottsville Group 0 25 50 KILOMETERS LTP k Kanawha Formation 40° LTP nr New River Formation LTP p Pocahontas Formation Mississippian Period Mmc Mauch Chunk Group Mbp Bluestone and Princeton Formations Ce Obrr Omc Mh Hinton Formation Obps Dmn Bluefield Formation Dbh Otbr Mbf MARYLAND LTP pv Osp Mg Greenbrier Group Smc Axis of Obs Mmp Maccrady and Pocono, undivided Burning Springs LTP a Mmc St Ce Mmcc Maccrady Formation anticline LTP d Om Dh Cwy Mp Pocono Group Qal Dhs Ch Devonian Period Mp Dohl LTP c Dmu Middle and Upper Devonian, undivided Obps Cw Dhs Hampshire Formation LTP m Dmn OHIO Ct Dch Chemung Group Omc Obs Dch Dbh Dbh Brailler and Harrell, undivided Stw Cwy LTP pv Ca Db Brallier Formation Obrr Cc 39° CPCc Dh Harrell Shale St Dmb Millboro Shale Mmc Dhs Dmt Mahantango Formation Do LTP d Ojo Dm Marcellus Formation Dmn Onondaga Group Om Lower Devonian, undivided LTP k Dhl Dohl Do Oriskany Sandstone Dmt Ot Dhl Helderberg Group LTP m VIRGINIA Qal Obr Silurian Period Dch Smc Om Stw Tonoloway, Wills Creek, and Williamsport Formations LTP c Dmb Sct Lower Silurian, undivided LTP a Smc McKenzie Formation and Clinton Group Dhl Stw Ojo Mbf Db St Tuscarora Sandstone Ordovician Period Ojo Juniata and Oswego Formations Dohl Mg Om Martinsburg Formation LTP nr Otbr Ordovician--Trenton and Black River, undivided 38° Mmcc Ot Trenton Group LTP k WEST VIRGINIA Obr Black River Group Omc Ordovician, middle calcareous units Mp Db Osp St. -

University of Michigan University Library
CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY , THE UNIVERSITY OF MICHIGAN VOL. XWI, No. 11, pp. 241-263 (2 pk., 5 figs.) OCTOBER9, 1962 A MISSISSIPPIAN FLORA FROM NORTHEASTERN UTAH AND ITS FAUNAL AND STRATIGRAPHIC RELATIONS BY CHESTER A. ARNOLD and WALTER SADLICK Published with aid from the Edward Pulteney Wright and Jean Davies Wright Expendable Trust Fund MUSEUM OF PfiEONTOLOGY THE UNIVERSITY OF MICHIGAN ANN ARBOR CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY Director: LEWISB. KELLUM The series of contributions from the Museum of Paleontology is a medium for the publication of papers based chiefly upon the collection in the Museum. When the number of pages issued is sufficient to make a volume, a title page and a table of contents will be sent to libraries on the mailing list, and to individuals upon request. A list of the separate papers may also be obtained. Correspondence should be directed to the Museum of Paleontology, The University of Michigan, Ann Arbor, Michigan. VOLS.11-XV. Parts of volumes may be obtained if available. VOLUMEXVI 1. Two Late Pleistocene Faunas from Southwestern Kansas, by Claude W. Hibbard and Dwight W. Taylor. Pages 1-223, with 16 plates. 2. North American Genera of the Devonian Rugose Coral Family Digonophylli- dae, by Erwin C. Stumm. Pages 225-243, with 6 plates. 3. Notes on Jaekelocystis hartleyi and Pseudocrinjtes gordoni, two Rhombi- feran Cystoids Described by Charles Schuchert in 1903, by Robert V. Kesling. Pages 245-273, with 8 plates. 4. Corals of the Traverse Group of Michigan. Part VI, Cladopora, Striatopora, and Thamnopora, by Erwin C. -

Note: Page Numbers in Italic Refer to Illustrations, Those in Bold Type Refer to Tables
Index Note: Page numbers in italic refer to illustrations, those in bold type refer to tables. Aachen-Midi Thrust 202, 203, 233, 235 Armorican affinities 132, 283 Acadian Armorican Massif 27, 29, 148, 390 basement 36 Armorican Terrane Assemblage 10, 13, 22 Orogeny 25 drift model 27-28 accommodation cycles 257, 265 magmatic rocks 75 accommodation space 265, 277 palaeolatitudes 28 acritarchs, Malopolska Massif 93 in Rheno-Hercynian Belt 42 advection, as heat source 378, 388 separation from Avalonia 49 African-European collision 22 tectonic m61ange 39 Air complex, palaeomagnetism 23, 25 Tepl/t-Barrandian Unit 44 Albersweiler Orthogneiss 40 terminology 132 Albtal Granite 48 Terrane Collage 132 alkali basalts 158 Ashgill, glacial deposits 28, 132, 133 allochthonous units, Rheno-Hercynian Belt 38 asthenosphere, upwelling 355, 376, 377 Alps asthenospheric source, metabasites 165 collisional orogeny 370 Attendorn-Elspe Syncline 241 see also Proto-Alps augen-gneiss 68 alteration, mineralogical 159 Avalon Terrane 87 Amazonian Craton 120, 122, 123, 147 Avalonia American Antarctic Ridge 167, 168, 170 and Amazonian Craton 120 Amorphognathus tvaerensis Zone 6 brachiopods 98 amphibolite facies metamorphism 41, 43, 67, 70 and Bronovistulian 110 Brunovistulian 106 collision with Armorica 298 Desnfi dome 179 collision with Baltica 52 MGCR 223 drift model 27 Saxo-Thuringia 283, 206 extent of 10 amphibolites, Bohemian Massif 156, 158 faunas 94 anatectic gneiss 45, 389 Gondwana derivation 22 anchimetamorphic facies 324 palaeolatitude 27 Anglo-Brabant Massif -
Stratigraphic Framework And
U.S. DEPARTMENT OF THE INTERIOR SCIENTIFIC INVESTIGATIONS MAP 2916 U.S. GEOLOGICAL SURVEY (SHEET 2 OF 2) Explanatory pamphlet accompanies map 82° 80° 78° 82° 80° 78° Table 1.—Wells used to construct cross section F–F '. Refer to figure 2 for well locations. Also shown are perforated intervals, initial production flow per day, and reservoir names. Lake Ontario [Reservoirs: C, “Clinton” sandstone; M, Medina sandstone; T, Tuscarora Sandstone. Oil and gas production terms: AF, after hydrofracturing; BO, barrels of oil; BW, barrels of water; MCFG, thousand cubic feet of gas; N, natural (no stimulation of reservoir); OH, open hole (no casing with perforations); P&A, plugged and abandoned; TA, temporarily abandoned; TO, trace of oil; TW, trace of water. Well that connects section F–F ' with section A–A' shown as 81, A–A' (54); number in parentheses refers to well 54 on section A–A' . A' Lake Ontario —, no data] A' Map American Township Date of Perforated or open hole Initial production flow Orleans Company Lease County/State Reservoirs no. Petroleum (7.5–min quadrangle) log run interval (depth in ft) of natural gas, oil, Niagara Institute no. and water (per day) Ontario Erie 1 34–089–22051 Southern Triangle Oil Co. No. 1 Swetnam Comm. Licking/Ohio Burlington 11–27–63 — P&A, 11–29–63; 1,200 MCFG, — N (from “Clinton” in nearby well No. 1 Houck, 6–30–1905) Lake Erie 2 34–089–22300 Lake Shore Pipeline Co. No. 1 Shipley Licking Burlington 12–13–65 — P&A, 12–12–65; 1,200 MCFG, — B N (from “Clinton” in nearby well No.