Dolomitization: Cambrian and Ordovician Formations in the Huron Domain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

59319647.Pdf
Article "Stratigraphic and Structural Framework of Upper Middle Ordovician Rocks in the Head Lake- Burleigh Falls Area of South-Central Ontario" Bruce V. Sanford Géographie physique et Quaternaire, vol. 47, n° 3, 1993, p. 253-268. Pour citer cet article, utiliser l'information suivante : URI: http://id.erudit.org/iderudit/032956ar DOI: 10.7202/032956ar Note : les règles d'écriture des références bibliographiques peuvent varier selon les différents domaines du savoir. Ce document est protégé par la loi sur le droit d'auteur. L'utilisation des services d'Érudit (y compris la reproduction) est assujettie à sa politique d'utilisation que vous pouvez consulter à l'URI https://apropos.erudit.org/fr/usagers/politique-dutilisation/ Érudit est un consortium interuniversitaire sans but lucratif composé de l'Université de Montréal, l'Université Laval et l'Université du Québec à Montréal. Il a pour mission la promotion et la valorisation de la recherche. Érudit offre des services d'édition numérique de documents scientifiques depuis 1998. Pour communiquer avec les responsables d'Érudit : [email protected] Document téléchargé le 12 février 2017 07:14 Géographie physique et Quaternaire, 1993, vol. 47, n° 3, p. 253-268, 15 fig. STRATIGRAPHIC AND STRUCTURAL FRAMEWORK OF UPPER MIDDLE ORDOVICIAN ROCKS IN THE HEAD LAKE- BURLEIGH FALLS AREA OF SOUTH- CENTRAL ONTARIO Bruce V. SANFORD, Independent Exploration Geologist,17 Meadowglade Gardens, Nepean, Ontario K2G 5J4. ABSTRACT Field investigations in the RÉSUMÉ Cadre stratigraphique et structu ZUSAM M EN FASSU NG Stratigraphischer Head Lake-Burleigh Falls area of south- ral des roches de l'Ordovicien moyen supé und struktureller Rahmen der Felsen aus central Ontario, that focused mainly on the rieur dans la région de Head Lake-Burleigh dem oberen mittleren Ordovizium in der Covey Hill(?), Shadow Lake, Gull River and Falls, au centre-sud de l'Ontario. -

Ground and Surface Water Monitoring Program Report
Phase 1 Geoscientific Desktop Preliminary Assessment, Processing and Interpretation of Borehole Geophysical Log and 2D Seismic Data MUNICIPALITY OF CENTRAL HURON, ONTARIO APM-REP-06144-0127 SEPTEMBER 2015 This report has been prepared under contract to the NWMO. The report has been reviewed by the NWMO, but the views and conclusions are those of the authors and do not necessarily represent those of the NWMO. All copyright and intellectual property rights belong to the NWMO. For more information, please contact: Nuclear Waste Management Organization 22 St. Clair Avenue East, Sixth Floor Toronto, Ontario M4T 2S3 Canada Tel 416.934.9814 Toll Free 1.866.249.6966 Email [email protected] www.nwmo.ca Phase 1 Geoscientific Desktop Preliminary Assessment, Processing and Interpretation of Borehole Geophysical Log and 2D Seismic Data Municipality of Central Huron Revision: 0 (Final) Prepared for: Nuclear Waste Management Organization 22 ST. Clair Avenue East, 6th Floor Toronto, Ontario M4T 2S3 Prepared by: Document ID: Central Huron_Final 2DSeismic BHGeophys Report_R0 NWMO Report Number: APM-REP-06144-0127 September, 2015 Interpretation of Borehole Geophysical Log and 2D Seismic Data Municipality of Central Huron Final Report Phase 1 Geoscientific Desktop Preliminary Assessment Processing and Interpretation of Borehole Geophysical Log and 2D Title: Seismic Data, Municipality of Central Huron Client: Nuclear Waste Management Organization Document ID: Central Huron_Final 2DSeismic BHGeophys Report_R0 Revision Number: 0 Date: September, 2015 Prepared -

Huiquan Guo Thesis
Long Term Two-Phase Flow Analysis of the Deep Low Permeability Rock at the Bruce DGR Site by Huiquan Guo A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Master of Applied Science in Civil Engineering Waterloo, Ontario, Canada, 2011 © Huiquan Guo 2011 I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. ii Abstract Abnormal pressures have been measured in the deep boreholes at the Bruce site, south- ern Ontario, where a deep geologic repository for low and intermediate level radioactive waste disposal has been proposed. The pressure regime in the stratigraphic units ex- hibits either higher than hydrostatic pressure (over-pressured) or lower than hydrostatic pressure (under-pressured) are considered to be abnormal. At the Bruce site, the Or- dovician sediments are under-pressured while the underlying Cambrian sandstone and the overlying Guelph carbonate are over-pressured. Hypotheses have been documented in literature to explain the phenomenon of abnormal pressures. These hypotheses include osmosis, glacial loading and deglaciation unloading, exhumation of overlying sediments, crustal flexure and the presence of an immiscible gas phase. Previous work on the Bruce site has shown that the under-pressures in the Ordovician limestone and shales could not be explained by glaciation and deglaciation or by saturated analyses. The presence of a gas phase in the Ordovician formations has been determined to be a reasonable cause of the under-pressure developed in the Ordovician shales and limestones at the Bruce site. -

Stratigraphic and Structural Framework of Upper Middle
Document generated on 09/26/2021 3:28 a.m. Géographie physique et Quaternaire Stratigraphic and Structural Framework of Upper Middle Ordovician Rocks in the Head Lake-Burleigh Falls Area of South-Central Ontario Cadre stratigraphique et structural des roches de l’Ordovicien moyen supérieur dans la région de Head Lake-Burleigh Falls, au centre-sud de l’Ontario Stratigraphischer und struktureller Rahmen der Felsen aus dem oberen mittleren Ordovizium in der Gegend von Head Lake-Burleigh Falls im südlichen Zentrum von Ontario Bruce V. Sanford La néotectonique de la région des Grands Lacs Article abstract Neotectonics of the Great Lakes area Field investigations in the Head Lake-Burleigh Falls area of south-central Volume 47, Number 3, 1993 Ontario, that focused mainly on the Covey Hill(?), Shadow Lake, Gull River and Coboconk formations along the Paleozoic escarpment, provided clear evidence URI: https://id.erudit.org/iderudit/032956ar of faulting. Observed and inferred structural deformation, coupled with faciès DOI: https://doi.org/10.7202/032956ar changes within the Shadow Lake and lower Gull River, point to a succession of basement movements during the Phanerozoic. These range from Hadrynian-Early Cambrian, through Middle Ordovician to post-late Middle See table of contents Ordovician times. Some of the earlier movements (Hadrynian-Early Cambrian to late Middle Ordovician) appear to be coincident with, and probably related to, plate tectonic events and the associated Taconian orogeny that were in Publisher(s) progress along the southeastern margins of the North American continent. Post-Middle Ordovician block faulting, on the other hand, may have been Les Presses de l'Université de Montréal triggered by any number of epeirogenic events related to late stage Taconian, Acadian or Alleghanian orogenies in Late Ordovician to Carboniferous times, ISSN or possibly to rifting associated with continental break-up and initiation of 0705-7199 (print) seafloor spreading processes in the early Mesozoic. -

Saugeen Shores
INITIAL SCREENING FOR SITING A DEEP GEOLOGICAL REPOSITORY FOR CANADA’S USED NUCLEAR FUEL The Corporation of the Town of Saugeen Shores INITIAL SCREENING FOR SITING A DEEP GEOLOGICAL REPOSITORY FOR CANADA’S USED NUCLEAR FUEL The Corporation of the Town of Saugeen Shores Prepared by: AECOM 300 – 300 Town Centre Boulevard 905 477 8400 tel Markham, ON, Canada L3R 5Z6 905 477 1456 fax www.aecom.com Project Number: 60247068-4 Date: September, 2012 The Corporation of the Town of Saugeen Shores Initial Screening for Siting a Deep Geological Repository for Canada’s Used Nuclear Fuel AECOM Signatures Report Prepared By: Robin Frizzell, M.Sc., P.Geo Senior Geoscientist Report Reviewed By: Robert E.J. Leech, P.Geo. Practice Lead, Environment The Corporation of the Town of Saugeen Shores Initial Screening for Siting a Deep Geological Repository for Canada’s Used Nuclear Fuel Executive Summary On May 14, 2012, the Corporation of the Town of Saugeen Shores expressed interest in learning more about the Nuclear Waste Management Organization’s (NWMO) site selection process to find an informed and willing community to host a deep geological repository for Canada’s used nuclear fuel (NWMO, 2010). This report summarizes the findings of an initial screening, conducted by AECOM, to evaluate the potential suitability of the Town of Saugeen Shores against five screening criteria using readily available information. The purpose of the initial screening is to identify whether there are any obvious conditions that would exclude the Town of Saugeen Shores from further consideration in the site selection process. The initial screening focused on the areas within the boundaries of the Town of Saugeen Shores. -

University of Michigan University Library
CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN VOL. 24, NO. 4, p. 23-30 (2 pls., 1 text-fig.) OCTOBER4, 1973 ORDOVICIAN VERTEBRATES FROM ONTARIO KATHLEEN ANNE LEHTOLA Michigan State University MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN ANN ARBOR CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY Director: ROBERTV. KESLING The series of contributions from the Museum of Paleontology is a medium for the publication of papers based chiefly upon the collection in the Museum. When the number of pages issued is sufficient to make a volume, a title page and a table of contents will be sent to libraries on the mailing list, and to individuals upon request. A list of the separate papers may also be obtained. Correspondence should be directed to the Museum of Paleontology, The University of Michigan, Ann Arbor, Michigan 48104. VOLS.2-23. Parts of volumes may be obtained if available. Price lists available upon inquiry. 1. A new species of Porocrinus from the Middle Ordovician Kimmswick Limestone of Missouri, by Robert V. Kesling. Pages 1-7, with 2 plates and 8 text-figures. 2. Strataster devonicus, a new brittle-star with unusual preservation from the Middle Devonian Silica Formation of Ohio, by Robert V. Kesling. Pages 9-15, with 2 plates and 3 text-figs. 3. Coccoliths and related calcareous nannofossils from the Upper Cretaceous Fencepost Limestone of northwestern Kansas, by John M. Huh and Charles I. Smith. Pages 17-22, with 2 plates. ORDOVICIAN VERTEBRATES FROM ONTARIO KATHLEEN ANNE LEHTOLA Michigan State University ABSTRACT-Vertebrate fossil fragments from the northeast part of St. -

Ordovician, Upper Sandbian-Katian) in Its Type Area: an Integrated Approach
Canadian Journal of Earth Sciences Revised stratigraphy of the middle Simcoe Group (Ordovician, upper Sandbian-Katian) in its type area: an integrated approach Journal: Canadian Journal of Earth Sciences Manuscript ID cjes-2018-0023.R2 Manuscript Type: Article Date Submitted by the 28-Dec-2018 Author: Complete List of Authors: Paton, Timothy; University of Tennessee, Knoxville, Earth and Planetary Sciences; University of Cincinnati, Geology Brett, Carlton;Draft University of Cincinnati, Geology Upper Ordovician, Kirkfield Formation, Bobcaygeon Formation, sequence Keyword: stratigraphy, chemostratigraphy Is the invited manuscript for consideration in a Special Not applicable (regular submission) Issue? : https://mc06.manuscriptcentral.com/cjes-pubs Page 1 of 50 Canadian Journal of Earth Sciences 1 Revised stratigraphy of the middle Simcoe Group (Ordovician, upper 2 Sandbian-Katian) in its type area: an integrated approach 3 4 Timothy R. Paton1 and Carlton E. Brett2 5 6 [email protected] 7 Department of Geology, University of Cincinnati, Cincinnati, OH 45221, USA 8 Currently [email protected] 9 Department of Earth and Planetary Sciences, University of Tennessee, Knoxville, Tennessee 10 37996-1410 11 12 [email protected] 13 Department of Geology, University of Cincinnati,Draft Cincinnati, OH 45221, USA 14 15 Timothy Robert Paton 16 1621 Cumberland Avenue, 602 Strong Hall, Knoxville TN 37996-1526 17 Phone: 901-292-7976 18 Fax: 865-974-2368 19 Email: [email protected] https://mc06.manuscriptcentral.com/cjes-pubs Canadian Journal of Earth Sciences Page 2 of 50 20 Abstract 21 The Upper Ordovician Bobcaygeon Formation of southern Ontario is a widespread unit 22 that spans the Sandbian-Katian stage boundary and contains exceptionally preserved invertebrate 23 fossil assemblages, including the famed ‘Kirkfield echinoderm fauna.’ However, the precise 24 correlation of this interval remains poorly understood. -

Ottawa-St. Lawrence Lowland, Southern Ontario
THESE TERMS GOVERN YOUR USE OF THIS DOCUMENT Your use of this Ontario Geological Survey document (the “Content”) is governed by the terms set out on this page (“Terms of Use”). By downloading this Content, you (the “User”) have accepted, and have agreed to be bound by, the Terms of Use. Content: This Content is offered by the Province of Ontario’s Ministry of Northern Development and Mines (MNDM) as a public service, on an “as-is” basis. Recommendations and statements of opinion expressed in the Content are those of the author or authors and are not to be construed as statement of government policy. You are solely responsible for your use of the Content. You should not rely on the Content for legal advice nor as authoritative in your particular circumstances. Users should verify the accuracy and applicability of any Content before acting on it. MNDM does not guarantee, or make any warranty express or implied, that the Content is current, accurate, complete or reliable. MNDM is not responsible for any damage however caused, which results, directly or indirectly, from your use of the Content. MNDM assumes no legal liability or responsibility for the Content whatsoever. Links to Other Web Sites: This Content may contain links, to Web sites that are not operated by MNDM. Linked Web sites may not be available in French. MNDM neither endorses nor assumes any responsibility for the safety, accuracy or availability of linked Web sites or the information contained on them. The linked Web sites, their operation and content are the responsibility of the person or entity for which they were created or maintained (the “Owner”). -

Paleozoic, Lake Simcoe
Ontario Geological Survey Open File Report 6011 Paleozoic Geology of the Northern Lake Simcoe Area, South-Central Ontario 2000 ONTARIO GEOLOGICAL SURVEY Open File Report 6011 Paleozoic Geology of the Northern Lake Simcoe Area, South-Central Ontario by D.K. Armstrong 2000 Parts of this publication may be quoted if credit is given. It is recommended that reference to this publication be made in the following form: Armstrong, D.K. 2000. Paleozoic geology of the northern Lake Simcoe area, south- central Ontario; Ontario Geological Survey, Open File Report 6011, 43p. e Queen’s Printer for Ontario, 2000 e Queen’s Printer for Ontario, 2000. Open File Reports of the Ontario Geological Survey are available for viewing at the Mines Library in Sudbury, at the Mines and Minerals Information Centre in Toronto, and at the regional Mines and Minerals office whose district includes the area covered by the report (see below). Copies can be purchased at Publication Sales and the office whose district includes the area covered by the report. Al- though a particular report may not be in stock at locations other than the Publication Sales office in Sudbury, they can generally be obtained within 3 working days. All telephone, fax, mail and e-mail orders should be directed to the Publica- tion Sales office in Sudbury. Use of VISA or MasterCard ensures the fastest possible service. Cheques or money orders should be made payable to the Minister of Finance. Mines and Minerals Information Centre (MMIC) Tel: (416) 314-3800 Macdonald Block, Room M2-17 1-800-665-4480(toll free inside Ontario) 900 Bay St. -

Stratigraphic Lexicon for Michigan
Stratigraphic Lexicon for Michigan AUTHORS Paul A. Catacosinos David B. Westjohn [Professor Emeritus, United States Geological Survey Delta College [Associate Professor (Adjunct), University Center, MI 48710] Michigan State University] 1001 Martingale Lane SE 6520 Mercantile Way #6 Albuquerque, NM 87123-4305 Lansing, MI 48911 William B. Harrison, III Mark S. Wollensak, CPG Professor, Department of Geosciences EarthFax Engineering, Inc. Western Michigan University 15266 Ann Drive Kalamazoo, MI 49008 Bath, MI 48808 Robert F. Reynolds Reynolds Geological, L.L.C. 504 Hall Blvd. Mason, MI, 48854 Bulletin 8 Lansing, Michigan, 2001 Geological Survey Division and the Michigan Basin Geological Society State of Michigan John Engler, Govenor Michigan Department of Environmental Quality Russell J. Harding, Director MDEQ Geological Survey Division, P O Box 30256, Lansing, MI 48909-7756 On the Internet @ HTTP://W WW .DEQ.STATE.MI.US/GSD Printed by Authority of Act 451, PA 1994 as amended The Michigan Department of Environmental Quality (MDEQ) will not discriminate Total number of copies printed ........... 1,000 against any individual or group on the basis of race, sex, religion, age, national origin, Total cost: .................................... $2,500.00 color, marital status, disability or political beliefs. Directed questions or concerns to the Cost per copy: ..................................... $2.50 MDEQ Office of Personnel Services, P.O. Box 30473, and Lansing, MI 48909 Page 2 - - Stratigraphic Lexicon for Michigan DEDICATION The authors gratefully dedicate this volume to the memories of Helen M. Martin and Muriel Tara Straight. This volume would not have been possible without their monumental reference work Bulletin 50, An Index of Helen Melville Martin Michigan Geology published by the Michigan Geological Survey in 1956. -

Index to the Geologic Names of North America
Index to the Geologic Names of North America GEOLOGICAL SURVEY BULLETIN 1056-B Index to the Geologic Names of North America By DRUID WILSON, GRACE C. KEROHER, and BLANCHE E. HANSEN GEOLOGIC NAMES OF NORTH AMERICA GEOLOGICAL SURVEY BULLETIN 10S6-B Geologic names arranged by age and by area containing type locality. Includes names in Greenland, the West Indies, the Pacific Island possessions of the United States, and the Trust Territory of the Pacific Islands UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1959 UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. SEATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, D.G. - Price 60 cents (paper cover) CONTENTS Page Major stratigraphic and time divisions in use by the U.S. Geological Survey._ iv Introduction______________________________________ 407 Acknowledgments. _--__ _______ _________________________________ 410 Bibliography________________________________________________ 410 Symbols___________________________________ 413 Geologic time and time-stratigraphic (time-rock) units________________ 415 Time terms of nongeographic origin_______________________-______ 415 Cenozoic_________________________________________________ 415 Pleistocene (glacial)______________________________________ 415 Cenozoic (marine)_______________________________________ 418 Eastern North America_______________________________ 418 Western North America__-__-_____----------__-----____ 419 Cenozoic (continental)___________________________________ -
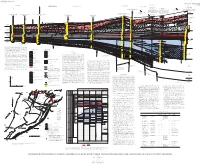
Stratigraphic Framework of Cambrian and Ordovician
U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY MISCELLANEOUS INVESTIGATIONS SERIES MAP I-2200 WOOSTER ARCH UNNAMED HINGE ZONE SE NORTHEASTERN END OF ROME TROUGH (MAIN BRANCH) ROME TROUGH (MINOR BRANCH) ALLEGHENY STRUCTURAL B' OHIO-WEST VIRGINIA HINGE ZONE 9t 11t FRONT Shell Oil Company California Company 10t No. 1 Snyder No. 1 Shade Mountain NW Deep Rock Oil Corporation Juniata Co., Pa. 8 Lycoming Co., Pa. No. A-1 Walizer B Texaco Incorporated and Marathon Oil Company No. 1 (Restored about 40 mi (64 km) southeastward to prethrusting A-A' Clinton Co., Pa. position using section C-C' from Berg and others (1980); bed Pennsylvania State Forest Tract 285 Composite outcrop section above drill hole Thrust faults showing about from Rones (1969) and Faill and Wells (1977) Composite outcrop section above drill hole thicknesses corrected for 45˚ dip above 4540 ft; gamma-ray Clinton Co., Pa 8 mi (12.9 km) shortening log above 4540 ft has not been corrected for dip) (Restored about 10 mi (16 km) southeastward from Thompson (1963), Rones (1969), Faill (Berg and others, 1980) Tie with to prethrusting position using section from Faill and Wells (1977), and Cotter (1982) Thrust faults showing about and Wells (1977); dip approximately horizontal, (Restored about 19 mi (30.6 km) southeast- LOWER (R.T. Ryder, unpub. data) unpub. Ryder, (R.T. FEET 8.5 mi (13.7 km) shortening thus, bed thickness correction unnecessary) ward to prethrusting position using section SILURIAN 7 10,000 from Faill and Wells (1977); bed thicknesses 1 (Faill and Wells, 1977) 2 4 Consolidated Gas Supply Corporation No.