Marine Ecology Progress Series 385:189
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Contribution of Wind-Generated Waves to Coastal Sea-Level Changes
1 Surveys in Geophysics Archimer November 2011, Volume 40, Issue 6, Pages 1563-1601 https://doi.org/10.1007/s10712-019-09557-5 https://archimer.ifremer.fr https://archimer.ifremer.fr/doc/00509/62046/ The Contribution of Wind-Generated Waves to Coastal Sea-Level Changes Dodet Guillaume 1, *, Melet Angélique 2, Ardhuin Fabrice 6, Bertin Xavier 3, Idier Déborah 4, Almar Rafael 5 1 UMR 6253 LOPSCNRS-Ifremer-IRD-Univiversity of Brest BrestPlouzané, France 2 Mercator OceanRamonville Saint Agne, France 3 UMR 7266 LIENSs, CNRS - La Rochelle UniversityLa Rochelle, France 4 BRGMOrléans Cédex, France 5 UMR 5566 LEGOSToulouse Cédex 9, France *Corresponding author : Guillaume Dodet, email address : [email protected] Abstract : Surface gravity waves generated by winds are ubiquitous on our oceans and play a primordial role in the dynamics of the ocean–land–atmosphere interfaces. In particular, wind-generated waves cause fluctuations of the sea level at the coast over timescales from a few seconds (individual wave runup) to a few hours (wave-induced setup). These wave-induced processes are of major importance for coastal management as they add up to tides and atmospheric surges during storm events and enhance coastal flooding and erosion. Changes in the atmospheric circulation associated with natural climate cycles or caused by increasing greenhouse gas emissions affect the wave conditions worldwide, which may drive significant changes in the wave-induced coastal hydrodynamics. Since sea-level rise represents a major challenge for sustainable coastal management, particularly in low-lying coastal areas and/or along densely urbanized coastlines, understanding the contribution of wind-generated waves to the long-term budget of coastal sea-level changes is therefore of major importance. -

Oceans in Motion TCS 2018
TCS Marine Biology C. Woodward Oceans in Motion TCS 2018 Oceans in Motion Oceans in Motion – TIDES (“la marea”) • Caused by gravitational forces between moon & Earth • Also influenced by sun, tilt of Earth, topography, and other factors DAILY TIDE CYCLE • 2 high tides, 2 low tides per 24 hrs (due to Earth’s rotation) • Tides get ~1 hr later each day gravitational pull of moon See Figs. Oceans in Motion - TIDES MONTHLY TIDE CYCLE • Due to moon’s orbit around Earth, and gravitational pull of moon & sun • 2 spring and 2 neap tides per month Fig. 3.33 Catherine Woodward 1 of 6 Tropical Conservation Semester 1 TCS Marine Biology C. Woodward Oceans in Motion TCS 2018 Oceans in Motion – WAVES (“las olas”) SURFACE WATER MOVEMENT is wind driven Waves = upper surface; move water only to ≈1/2 wavelength (λ) Nybakken Fig 1.10 See C&H Fig. 3.27 Oceans in Motion Water movement is circular But circles not closed, especially in big waves and shallow water. Stoke’s Drift = displacement of water in the direction of wave movement Oceans in Motion SWELLS Wave size determined by: • Wind speed • Fetch • Duration See C&H Fig. 3.29 Longer waves move faster Catherine Woodward 2 of 6 Tropical Conservation Semester 2 TCS Marine Biology C. Woodward Oceans in Motion TCS 2018 Oceans in Motion – CURRENTS (“la corriente”) NEAR-SHORE CURRENTS • Created by wind (= waves) and shore topography • Longshore current, undertow, rip current Undertow: The seaward return of water along the bottom underneath breaking waves Oceans in Motion NEAR-SHORE CURRENTS • Created by wind (= waves) and shore topography • Longshore current, undertow, rip current Longshore current: Results when waves hit shore at an angle, pushing water and material down the shore. -

Momentum Balance Across a Barrier Reef Damien Sous, G
Momentum balance across a barrier reef Damien Sous, G. Dodet, F. Bouchette, M. Tissier To cite this version: Damien Sous, G. Dodet, F. Bouchette, M. Tissier. Momentum balance across a barrier reef. Journal of Geophysical Research. Oceans, Wiley-Blackwell, 2020, 125 (2), pp.e2019JC015503. 10.1029/2019JC015503. hal-02520270 HAL Id: hal-02520270 https://hal.archives-ouvertes.fr/hal-02520270 Submitted on 4 Aug 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. RESEARCH ARTICLE Momentum Balance Across a Barrier Reef 10.1029/2019JC015503 D. Sous1,2 , G. Dodet3 , F. Bouchette4,5 , and M. Tissier6 Key Points: 1 • The first complete evaluation of Mediterranean Institute of Oceanography (MIO), Université de Toulon, Aix Marseille Université, CNRS, IRD, La Garde, momentum balance over a reef France, 2Univ Pau & Pays Adour / E2S UPPA, Chaire HPC-Waves, Laboratoire des Sciences de lIngénieur Appliques a la barrier from measurements and Méchanique et au Génie Electrique Fédération IPRA, ANGLET, France, 3IFREMER, Univ. Brest, CNRS, IRD, Laboratoire numerical modeling -

Shaping the Beach, One Wave at a Time New Research Is Deciphering How Currents, Waves, and Sands Change Our Shorelines
http://oceanusmag.whoi.edu/v43n1/raubenheimer.html Shaping the Beach, One Wave at a Time New research is deciphering how currents, waves, and sands change our shorelines By Britt Raubenheimer, Associate Scientist nearshore region—the stretch of sand, for a beach to erode or build up. Applied Ocean Physics & Engineering Dept. rock, and water between the dry land be- Understanding beaches and the adja- Woods Hole Oceanographic Institution hind the beach and the beginning of deep cent nearshore ocean is critical because or years, scientists who study the water far from shore. To comprehend and nearly half of the U.S. population lives Fshoreline have wondered at the appar- predict how shorelines will change from within a day’s drive of a coast. Shoreline ent fickleness of storms, which can dev- day to day and year to year, we have to: recreation is also a significant part of the astate one part of a coastline, yet leave an • decipher how waves evolve; economy of many states. adjacent part untouched. One beach may • determine where currents will form For more than a decade, I have been wash away, with houses tumbling into the and why; working with WHOI Senior Scientist Steve sea, while a nearby beach weathers a storm • learn where sand comes from and Elgar and colleagues across the coun- without a scratch. How can this be? where it goes; try to decipher patterns and processes in The answers lie in the physics of the • understand when conditions are right this environment. Most of our work takes A Mess of Physics Near the Shore Many forces intersect and interact in the surf and swash zones of the coastal ocean, pushing sand and water up, down, and along the coast. -

Comparative Perspectives on the Rise of the Brazilian Novel COMPARATIVE LITERATURE and CULTURE
Comparative Perspectives on the Rise of the Brazilian Novel COMPARATIVE LITERATURE AND CULTURE Series Editors TIMOTHY MATHEWS AND FLORIAN MUSSGNUG Comparative Literature and Culture explores new creative and critical perspectives on literature, art and culture. Contributions offer a comparative, cross- cultural and interdisciplinary focus, showcasing exploratory research in literary and cultural theory and history, material and visual cultures, and reception studies. The series is also interested in language-based research, particularly the changing role of national and minority languages and cultures, and includes within its publications the annual proceedings of the ‘Hermes Consortium for Literary and Cultural Studies’. Timothy Mathews is Emeritus Professor of French and Comparative Criticism, UCL. Florian Mussgnug is Reader in Italian and Comparative Literature, UCL. Comparative Perspectives on the Rise of the Brazilian Novel Edited by Ana Cláudia Suriani da Silva and Sandra Guardini Vasconcelos First published in 2020 by UCL Press University College London Gower Street London WC1E 6BT Available to download free: www.uclpress.co.uk Collection © Editors, 2020 Text © Contributors, 2020 The authors have asserted their rights under the Copyright, Designs and Patents Act 1988 to be identified as the authors of this work. A CIP catalogue record for this book is available from The British Library. This book is published under a Creative Commons 4.0 International licence (CC BY 4.0). This licence allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work). -

Leslie Silko E James Redfield, Mentores De Uma Nova Consciência
Zaida Pinto Ferreira Leslie Silko e James Redfield, Mentores de uma Nova Consciência: da Fragmentação à Unidade Tese de Doutoramento em Literatura Especialidade em Literatura Norte-Americana UNIVERSIDADE ABERTA LISBOA 2013 Zaida Pinto Ferreira Leslie Silko e James Redfield, Mentores de uma Nova Consciência: da Fragmentação à Unidade Tese de Doutoramento apresentada à Universidade Aberta e realizada sob a orientação da Ex.ma Senhora Professora Doutora Maria Filipa Palma dos Reis UNIVERSIDADE ABERTA LISBOA 2013 A todos os Mestres que me inspiraram. Agradecimentos A elaboração desta dissertação de doutoramento contou com preciosos contributos que pretendo destacar. Assim, agradeço à Professora Doutora Filipa Palma dos Reis, minha orientadora, a disponibilidade, a dedicação, o apoio, e a leitura atenta e rigorosa que dedicou a este estudo, contribuindo para ampliar a minha visão sobre o tema. O seu empenho foi crucial para o resultado final deste trabalho. Expresso o meu agradecimento à Ana Maria Costa pelas preciosas sugestões que me facultou e pela leitura crítica, atenta e rigorosa que lhe mereceu o meu texto. Agradeço ainda às minhas amigas Anabela Sardo e Isa Severino pelo estímulo constante ao longo deste percurso, pelas ajudas que deram a este estudo, contribuindo para melhorar alguns aspectos. De igual modo, expresso o meu reconhecimento aos meus amigos que não preciso nomear, porque, de modo indirecto, concorreram para o sucesso do meu trabalho, com o seu apreço e amizade. Dedico uma mensagem especial aos meus filhos, Tiago, André, à minha mãe, ao meu irmão e à Mila, pois sem o saberem, deram-me um extremo alento para prosseguir esta travessia. -

POETRY IS NOT MADE of WORDS: (C) Copyright by Carmen Caliz
POETRY IS NOT MADE OF WORDS: A STUDY OF AESTHETICS OF THE BORDERLANDS IN GLORIA ANZALDOA AND MARLENE NOURBESE PHILIP Carmen Caliz-Montoro A thesis submitted in conformity with the requirements for the degree of Ph-D. Graduate Department of Comparative Literature University of Toronto (c) Copyright by Carmen Caliz-Montoro 1996. Acquisitions and Acquisiions et Bibliographic Services services bibliographiques The author has granted a non- L'auteur a accorde me Licence non exclusive licence allowing the exclusive permettant a la National Library of Canada to Blaliothkque nationale du Canada de reproduce, loan, distriiute or sell reproduke, pr&er, distriiuer ou copies of this thesis m microform, vendre des copies de cette these sous paper or electronic formats. la fonne de microfichdfilm, de reproduction sur papier ou sur fonnat electronique. The author retains ownership of the L'autem conserve la propriete du copyright in this thesis. Neither the droit d'auteur qui protege cette these. thesis nor substantial extracts fkom it Ni h these ni des erctraits substantiels may be printed or otherwise de celle-ci ne doivent &re imprimes reproduced without the author's ou autrement reproduits sans son permission. autorisation. POETRY IS NOT MADE OF WORDS: A STUDY OF AESTHETICS OF THE BORDERLANDS IN GLORIA ANZALDOA AND MARLENE NOURBESE PHILIP Carmen Caliz-Montoro, Ph.D. University of Toronto, 1996. Supervisor: Prof. Frederick I. Case ABSTRACT This thesis explores an alternative critical approach as a way to overcome the limitations of North American cultural criticism in dealing with literature from the borderlands. Literatures that are in between two or more cultures are not we11 served by one established cultural context. -
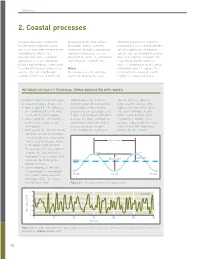
2. Coastal Processes
CHAPTER 2 2. Coastal processes Coastal landscapes result from by weakening the rock surface and driving nearshore sediment the interaction between coastal to facilitate further sediment transport processes. Wind and tides processes and sediment movement. movement. Biological, biophysical are also significant contributors, Hydrodynamic (waves, tides and biochemical processes are and are indeed dominant in coastal and currents) and aerodynamic important in coral reef, salt marsh dune and estuarine environments, (wind) processes are important. and mangrove environments. respectively, but the action of Weathering contributes significantly waves is dominant in most settings. to sediment transport along rocky Waves Information Box 2.1 explains the coasts, either directly through Ocean waves are the principal technical terms associated with solution of minerals, or indirectly agents for shaping the coast regular (or sinusoidal) waves. INFORMATION BOX 2.1 TECHNICAL TERMS ASSOCIATED WITH WAVES Important characteristics of regular, Natural waves are, however, wave height (Hs), which is or sinusoidal, waves (Figure 2.1). highly irregular (not sinusoidal), defined as the average of the • wave height (H) – the difference and a range of wave heights highest one-third of the waves. in elevation between the wave and periods are usually present The significant wave height crest and the wave trough (Figure 2.2), making it difficult to off the coast of south-west • wave length (L) – the distance describe the wave conditions in England, for example, is, on between successive crests quantitative terms. One way of average, 1.5m, despite the area (or troughs) measuring variable height experiencing 10m-high waves • wave period (T) – the time from is to calculate the significant during extreme storms. -

Experimental Study on the Influence of an Artificial Reef on Cross-Shore
water Article Experimental Study on the Influence of an Artificial Reef on Cross-Shore Morphodynamic Processes of a Wave-Dominated Beach Yue Ma 1 , Cuiping Kuang 1,*, Xuejian Han 1, Haibo Niu 2, Yuhua Zheng 1 and Chao Shen 1 1 College of Civil Engineering, Tongji University, Shanghai 200092, China; [email protected] (Y.M.); [email protected] (X.H.); [email protected] (Y.Z.); [email protected] (C.S.) 2 Department of Engineering, Dalhousie University, Truro, NS B2N 5E3, Canada; [email protected] * Correspondence: [email protected] Received: 4 August 2020; Accepted: 18 October 2020; Published: 21 October 2020 Abstract: Artificial reefs are being implemented around the world for their multi-functions including coastal protection and environmental improvement. To better understand the hydrodynamic and morphodynamic roles of an artificial reef (AR) in beach protection, a series of experiments were conducted in a 50 m-long wave flume configured with a 1:10 sloping beach and a model AR (1.8 m long 0.3 m high) with 0.2 m submergence depth. Five regular and five irregular wave × conditions were generated on two types of beach profiles (with/without model AR) to study the cross-shore hydrodynamic and morphological evolution process. The influences of AR on the processes are concluded as follows: (1) AR significantly decreases the incident wave energy, and its dissipation effect differs for higher and lower harmonics under irregular wave climates; (2) AR changes the cross-shore patterns of hydrodynamic factors (significant wave height, -

Formulation of the Undertow Using Linear Wave Theory
Formulation of the undertow using linear wave theory Guannel, G., & Özkan-Haller, H. T. (2014). Formulation of the undertow using linear wave theory. Physics of Fluids, 26(5), 056604. doi:10.1063/1.4872160 10.1063/1.4872160 American Institute of Physics Publishing Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse PHYSICS OF FLUIDS 26, 056604 (2014) Formulation of the undertow using linear wave theory G. Guannel1,a) and H. T. Ozkan-Haller¨ 2 1School of Civil and Construction Engineering, Oregon State University, Corvallis, Oregon 97331, USA 2College of Earth, Ocean, and Atmospheric Sciences, Oregon State University, Corvallis, Oregon 97331, USA (Received 6 September 2013; accepted 7 February 2014; published online 13 May 2014) The undertow is one of the most important mechanisms for sediment transport in nearshore regions. As such, its formulation has been an active subject of research for at least the past 40 years. Still, much debate persists on the exact nature of the forcing and theoretical expression of this current. Here, assuming linear wave theory and keeping most terms in the wave momentum equations, a solution to the undertow in the surf zone is derived, and it is shown that it is unique. It is also shown that, unless they are erroneous, most solutions presented in the literature are identical, albeit simplified versions of the solution presented herein. Finally, it is demonstrated that errors in past derivations of the undertow profile stem from inconsistencies between (1) the treatment of advective terms in the momentum equations and the wave action equation, (2) the expression of the mean current equation and the surface shear stress, and (3) the omission of bottom shear stress in the momentum equation. -

OCEANOGRAPHY STUDY GUIDE Chapter 2 Section 1 1
OCEANOGRAPHY STUDY GUIDE Chapter 2 Section 1 1. Most abundant salt in ocean. Sodium chloride; NaCl 2. Amount of Earth covered by Water 71% 3. Four oceans: What are they? Atlantic, Pacific, Arctic, Indian . Largest? Pacific . Smallest? Arctic . Locations of each? Atlantic – between the Americas and Africa; Pacific – between the Americas and Asia; Indian – beneath Asia, to the east of Africa; Arctic- above Russia, Europe, and Canada Chapter 2 Section 1 4. What is Salinity? The amount of dissolved salt in a given amount of water 5. How is salinity increased in the ocean? Evaporation, Freezing, More runoff following erosion . How is it decreased? Increased rainfall, Melting of ice; Increase of freshwater runoff 6. What influences density of water? Changes in temperature and salinity 7. Basics parts of the water cycle. Condensation – water goes from gas to a liquid Evaporation – water goes from a liquid to a gas Precipitation – water becomes too heavy and falls out of the atmosphere 8. What is the ocean’s most important function? EXPLAIN! To absorb the radiation from the sun. This helps regulate to temperatures on land, preventing large temperature fluctuations. Chapter 2 Section 2 1. How do scientists study the ocean floor? SONAR, satellite, submersibles 2. Major regions of the ocean floor • Continental Margin and Deep-ocean basin Where are these regions located? Can you describe them? Continental Shelf – located in continental margin; closest to shoreline Continental Slope – located in the margin; steep slope Continental Rise – located in the margin; gentle slope leading into basin Abyssal Plain – part of basin; large, flat area of ocean floor Ocean Trench – part of basin; deepest areas of the ocean floor; found at subduction zones Sea Mount – part of the basin; mountain on the ocean floor; can become a guyot or volcanic island Chapter 3 Section 1 1. -

110 Misconceptions About the Ocean Oceanography B Y R O B Ert J
This article has This been published in or collective redistirbution of any portion of this article by photocopy machine, reposting, or other means is permitted only with the approval of The approval portionthe ofwith any permitted articleonly photocopy by is of machine, reposting, this means or collective or other redistirbution EDUCATIO N 110 Misconceptions About the Ocean Oceanography B Y R O B erT J . F E LL er , Volume 20, Number 4, a quarterly journal of The 20, Number 4, a quarterly , Volume Misconceptions impede student learn- at the University of Washington in sum- classes I’ve taught since 1970, one would ing, especially in science. As teachers mer 1970 (taught by Eddy Carmack, think that few misconceptions would go of earth science, we would be wise to by the way), I have come across stu- unheard or unread on tests, but this is identify misconceptions whenever pos- dent misconceptions too numerous to simply not the case. I still confront new sible before launching new topics in count. The very first one came during ones all the time. Thanks to the current our oceanography courses. A good way a one-on-one laboratory write-up help crop of students in my Fundamentals to do this is to pose creative, multiple- session in this class. The student was a of Biological Oceanography class, O ceanography Society. Society. ceanography choice, PowerPoint questions that freshman nonoceanography, nonscience a few more were added to the list can be answered anonymously using major who wondered why people in just this semester. student-response systems, “clickers,” in the Southern Hemisphere didn’t fall What you do with misconceptions can large introductory classes (Beatty et al., off the earth.