Jellyfish (Cnidaria/Ctenophora)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Temperature in Survival of the Polyp Stage of the Tropical Rhizostome Jelly®Sh Cassiopea Xamachana
Journal of Experimental Marine Biology and Ecology, L 222 (1998) 79±91 The role of temperature in survival of the polyp stage of the tropical rhizostome jelly®sh Cassiopea xamachana William K. Fitt* , Kristin Costley Institute of Ecology, Bioscience 711, University of Georgia, Athens, GA 30602, USA Received 27 September 1996; received in revised form 21 April 1997; accepted 27 May 1997 Abstract The life cycle of the tropical jelly®sh Cassiopea xamachana involves alternation between a polyp ( 5 scyphistoma) and a medusa, the latter usually resting bell-down on a sand or mud substratum. The scyphistoma and newly strobilated medusa (5 ephyra) are found only during the summer and early fall in South Florida and not during the winter, while the medusae are found year around. New medusae originate as ephyrae, strobilated by the polyp, in late summer and fall. Laboratory experiments showed that nematocyst function, and the ability of larvae to settle and metamorphose change little during exposure to temperatures between 158C and up to 338C. However, tentacle length decreased and ability to transfer captured food to the mouth was disrupted at temperatures # 188C. Unlike temperate-zone species of scyphozoans, which usually over-winter in the polyp or podocyst form when medusae disappear, this tropical species has cold-sensitive scyphistomae and more temperature-tolerant medusae. 1998 Elsevier Science B.V. Keywords: Scyphozoa; Jelly®sh; Cassiopea; Temperature; Life history 1. Introduction The rhizostome medusae of Cassiopea xamachana are found throughout the Carib- bean Sea, with their northern limit of distribution on the southern tip of Florida. Unlike most scyphozoans these jelly®sh are seldom seen swimming, and instead lie pulsating bell-down on sandy or muddy substrata in mangroves or soft bottom bay habitats, giving rise to the common names ``mangrove jelly®sh'' or ``upside-down jelly®sh''. -

Treatment of Lion´S Mane Jellyfish Stings- Hot Water Immersion Versus Topical Corticosteroids
THE SAHLGRENSKA ACADEMY Treatment of Lion´s Mane jellyfish stings- hot water immersion versus topical corticosteroids Degree Project in Medicine Anna Nordesjö Programme in Medicine Gothenburg, Sweden 2016 Supervisor: Kai Knudsen Department of Anesthesia and Intensive Care Medicine 1 CONTENTS Abstract ................................................................................................................................................... 3 Introduction ............................................................................................................................................. 3 Background ............................................................................................................................................. 4 Jellyfish ............................................................................................................................................... 4 Anatomy .......................................................................................................................................... 4 Nematocysts .................................................................................................................................... 4 Jellyfish in Scandinavian waters ......................................................................................................... 5 Lion’s Mane jellyfish, Cyanea capillata .......................................................................................... 5 Moon jelly, Aurelia aurita .............................................................................................................. -

Fish Welfare on Scotland's Salmon Farms
FISH WELFARE ON SCOTLAND’S SALMON FARMS A REPORT BY ONEKIND Lorem ipsum CONTENTS 1 INTRODUCTION 2 6.3.1 Increased aggression 26 6.3.2 Increased spread of disease and parasites 26 2 SALMON SENTIENCE 6.3.2 Reduced water quality 26 AND INDIVIDUALITY 4 6.3.4 Issues with low stocking densities 27 2.1 Fish sentience 5 6.4 Husbandry 27 2.2 Atlantic salmon as individuals 5 6.4.1 Handling 27 6.4.2 Crowding 28 3 ATLANTIC SALMON LIFE CYCLE 6 6.4.3 Vaccination 28 3.1 Life cycle of wild salmon 6 6.5 Transportation 28 3.2 Life cycle of farmed Alantic Salmon 6 6.6 Failed smolts 29 6.7 Housing 20 4 SALMON FARMING IN SCOTLAND 8 6.8 Slaughter 31 5 KEY WELFARE ISSUES 10 7 MARINE WILDLIFE WELFARE IMPACTS 32 5.1 High mortality rates 10 7.1 Wild salmon and trout 32 5.2 Sea lice 11 7.2 Fish caught for salmon food 33 5.2.1 How do sea lice compromise 7.3 Seals 33 the welfare of farmed salmon? 11 7.4 Cetaceans 34 5.2.2 How are sea lice levels monitored? 12 7.5 Crustaceans 34 5.2.3 How severe is sea lice infestation in Scotland? 13 8 FUTURE CHALLENGES 35 5.3 Disease 14 8.1 Closed containment 35 5.3.1 Amoebic Gill Disease 15 8.2 Moving sites offshore 35 5.3.2 Cardiomyopathy Syndrome 15 5.3.3 Infectious salmon anaemia 15 9 ACCREDITATION SCHEMES 5.3.4 Pancreas disease 15 AND STANDARDS 36 5.4 Treatment for sea lice and disease 16 9.1 Certification in Scotland 36 5.4.1 Thermolicer 17 9.2 What protection do standards provide salmon? 36 5.4.2 Hydrolicer 17 9.2.1 Soil Association Organic standards 36 5.4.3 Hydrogen peroxide 17 9.2.2 RSPCA Assured 36 5.5 Cleaner fish 18 -

Predatory Impact of Aurelia Aurita on the Zooplankton Community in The
Barz and Hirche: Predatory impact of Aurelia aurita on the zooplankton community in the Bornholm Basin (central Baltic) CM 2004/J: 12 ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯ Not to be cited without prior reference to the authors CM 2004/J:12 Predatory impact of Aurelia aurita on the zooplankton community in the Bornholm Basin (central Baltic) Kristina Barz and Hans-Jürgen Hirche Alfred-Wegener-Institute for Polar and Marine Research Columbusstrasse, 27568 Bremerhaven, Germany tel.: +49 471 4831 1324, fax: +49 471 4831 1918 [email protected] Abstract The annual cycle of abundance and vertical distribution of the scyphozoan medusa Aurelia aurita was studied in the Bornholm Basin (central Baltic) in 2002. Seasonal changes in prey composition and predatory impact were investigated by analysing stomach contents. The dominant prey organisms of A. aurita were several cladoceran species (Bosmina coregoni maritima, Evadne nordmanni and Podon spp.). They represented up to 93% of the food organisms. Bivalve larvae and copepods (Centropages hamatus, Temora longicornis, Acartia spp., Pseudocalanus acuspes, Eurytemora hirundoides and Oithona similis) were less frequently found. Nauplii and copepodites I-III were not eaten by the medusae neither were fish eggs and larvae used as prey. Based on average medusae and zooplankton abundance, the predatory impact was very low. In August, when mean abundance of A. aurita was highest, only 0.1% of the copepod and 0.54% of the cladoceran standing stock were eaten per day. However in regions -

Hydrozoa of the Eurasian Arctic Seas 397 S
THE ARCTIC SEAS CI imatology, Oceanography, Geology, and Biology Edited by Yvonne Herman IOm51 VAN NOSTRAND REINHOLD COMPANY ~ -----New York This work relates to Department of the Navy Grant NOOOI4-85- G-0252 issued by the Office of Naval Research. The United States Government has a royalty-free license throughout the world in all copyrightable material contained herein. Copyright © 1989 by Van Nostrand Reinhold Softcover reprint of the hardcover 1st edition 1989 Library of Congress Catalog Card Number 88-33800 ISBN-13 :978-1-4612-8022-4 e-ISBN-13: 978-1-4613-0677-1 DOI: 10.1007/978-1-4613-0677-1 All rights reserved. No part of this work covered by the copyright hereon may be reproduced or used in any form or by any means-graphic, electronic, or mechanical, including photocopying, recording, taping, or information storage and retrieval systems-without written permission of the publisher. Designed by Beehive Production Services Van Nostrand Reinhold 115 Fifth Avenue New York, New York 10003 Van Nostrand Reinhold (International) Limited 11 New Fetter Lane London EC4P 4EE, England Van Nostrand Reinhold 480 La Trobe Street Melbourne, Victoria 3000, Australia Nelson Canada 1120 Birchmount Road Scarborough, Ontario MIK 5G4, Canada 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 Library of Congress Cataloging in Publication Data The Arctic Seas. Includes index. 1. Oceanography-Arctic Ocean. 2. Geology-ArctiC Ocean. 1. Herman, Yvonne. GC401.A76 1989 551.46'8 88-33800 ISBN-13: 978-1-4612-8022-4 For Anyu Contents Preface / vii Contributors / ix 1. -

Pdf) and Their Values Are Plotted Against Temperature in Fig
Vol. 510: 255–263, 2014 MARINE ECOLOGY PROGRESS SERIES Published September 9 doi: 10.3354/meps10799 Mar Ecol Prog Ser Contribution to the Theme Section ‘Jellyfish blooms and ecological interactions’ FREEREE ACCESSCCESS Body size reduction under starvation, and the point of no return, in ephyrae of the moon jellyfish Aurelia aurita Zhilu Fu1, Masashi Shibata1, Ryosuke Makabe2, Hideki Ikeda1, Shin-ichi Uye1,* 1Graduate School of Biosphere Science, Hiroshima University, 4-4 Kagamiyama 1 Chome, Higashi-Hiroshima 739−8528, Japan 2Faculty of Science and Engineering, Ishinomaki Senshu University, 1 Shinmito Minamisakai, Ishinomaki 986-8580, Japan ABSTRACT: Scyphozoan ephyrae need to start feeding before their endogenous nutritional reserves run out, and the success of feeding and growth is crucial to their recruitment into the medusa population. To evaluate starvation resistance in first-feeding ephyrae of the moon jellyfish Aurelia aurita s.l., we determined their point of no return (PNR50), i.e. days of starvation after which 50% of ephyrae die even if they then feed. PNR50 values were 33.8, 38.4 and 58.6 d at 15, 12 and 9°C, respectively. Before reaching PNR50, the ephyrae showed significant body size reduc- tion: ca. 30 and 50% decrease in disc diameter and carbon content, respectively. These PNR50 val- ues are nearly 1 order of magnitude longer than those of larval marine molluscs, crustaceans and fishes, which is attributable to the ephyra’s extremely low metabolic (i.e. respiration) rate relative to its copious carbon reserves. Such a strong endurance under prolonged starvation is likely an adaptive strategy for A. aurita ephyrae, the release of which is programmed to occur during the annual period of lowest temperatures, allowing them to cope with the concomitant seasonal food scarcity. -

Clearance Rates of Jellyfish and Their Potential Predation Impact on Zooplankton and Fish Larvae in a Neritic Ecosystem (Limfjorden, Denmark)
MARINE ECOLOGY PROGRESS SERIES Vol. 304: 117–131, 2005 Published December 8 Mar Ecol Prog Ser Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden, Denmark) Lars Johan Hansson1,*, Ole Moeslund2, Thomas Kiørboe1, Hans Ulrik Riisgård2 1Danish Institute for Fisheries Research, Kavalergården 6, 2920 Charlottenlund, Denmark 2Marine Biological Research Centre (University of Southern Denmark) Hindsholmvej 11, 5300 Kerteminde, Denmark ABSTRACT: Clearance rates of the hydromedusae Sarsia tubulosa, Rathkea octopunctata and Bougainvillea superciliaris and the scyphomedusa Aurelia aurita were measured in the laboratory. Gut contents analyses of A. aurita were also collected in situ and subsequently used for estimation of clearance rate. The clearance rate of A. aurita varied widely with prey organisms. Large crustacean prey with low escape capabilities (Artemia salina nauplii and cirripede larvae) were cleared at high rates, whereas copepodites were cleared at lower rates, and clearance rates of small bivalve larvae and copepod nauplii were comparatively low. These data were used to assess the impact of jellyfish preda- tion upon zooplankton and fish larvae in Limfjorden, Denmark. Repeated sampling of zooplankton, fish larvae and medusae was undertaken during the first half of 2003. Nine taxa of hydromedusae and 4 taxa of scyphomedusae were identified. Abundance estimates were combined with estimated clear- ance rates of individual medusae to calculate potential jellyfish-induced mortality on prey in Limfjor- den. Copepoda was used as a model prey group to estimate the collective predation impact by all medusae. Medusa species with unknown clearance potential were given assumed clearance rate val- ues, but the collective predation potential by these species was evaluated to be small. -

The Puzzling Occurrence of the Upside-Down Jellyfish Cassiopea
ZOOLOGIA 37: e50834 ISSN 1984-4689 (online) zoologia.pensoft.net RESEARCH ARTICLE The puzzling occurrence of the upside-down jellyfishCassiopea (Cnidaria: Scyphozoa) along the Brazilian coast: a result of several invasion events? Sergio N. Stampar1 , Edgar Gamero-Mora2 , Maximiliano M. Maronna2 , Juliano M. Fritscher3 , Bruno S. P. Oliveira4 , Cláudio L. S. Sampaio5 , André C. Morandini2,6 1Departamento de Ciências Biológicas, Laboratório de Evolução e Diversidade Aquática, Universidade Estadual Paulista “Julio de Mesquita Filho”. Avenida Dom Antônio 2100, 19806-900 Assis, SP, Brazil. 2Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo. Rua do Matão, Travessa 14 101, 05508-090 São Paulo, SP, Brazil. 3Instituto do Meio Ambiente de Alagoas. Avenida Major Cícero de Góes Monteiro 2197, 57017-515 Maceió, AL, Brazil. 4Instituto Biota de Conservação, Rua Padre Odilon Lôbo, 115, 57038-770 Maceió, Alagoas, Brazil 5Laboratório de Ictiologia e Conservação, Unidade Educacional de Penedo, Universidade Federal de Alagoas. Avenida Beira Rio, 57200-000 Penedo, AL, Brazil. 6Centro de Biologia Marinha, Universidade de São Paulo. Rodovia Manoel Hypólito do Rego, km 131.5, 11612-109 São Sebastião, SP, Brazil. Corresponding author: Sergio N. Stampar ([email protected]) http://zoobank.org/B879CA8D-F6EA-4312-B050-9A19115DB099 ABSTRACT. The massive occurrence of jellyfish in several areas of the world is reported annually, but most of the data come from the northern hemisphere and often refer to a restricted group of species that are not in the genus Cassiopea. This study records a massive, clonal and non-native population of Cassiopea and discusses the possible scenarios that resulted in the invasion of the Brazilian coast by these organisms. -

The Jellyfish Fishery in Mexico
Vol.4, No.6A, 57-61 (2013) Agricultural Sciences http://dx.doi.org/10.4236/as.2013.46A009 The jellyfish fishery in Mexico Juana López-Martínez*, Javier Álvarez-Tello Centro de Investigaciones Biológicas del Noroeste, S.C. (CIBNOR), Unidad Sonora, Campus Guaymas, Guaymas, México; *Corresponding Author: [email protected] Received 26 April 2013; revised 26 May 2013; accepted 15 June 2013 Copyright © 2013 Juana López-Martínez, Javier Álvarez-Tello. This is an open access article distributed under the Creative Com- mons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT ure 1), because some species secrete painful neurotoxic, even deadly, venom. Globally there has been a slight Jellyfish has been captured in Asia for 1700 increase of individuals in this group of organisms during years, and it has been considered a delicacy. the last decades [1], but in some countries as Australia, Since the 70s important jellyfish fisheries have jellyfish is considered a plague, so in response the gov- developed in several parts of the world, with ernment developed programs to control them. Among the catches increasing exponentially, reaching possible causes of the increase of jellyfish population, an 500,000 tons per year in the mid-nineties. In increase in water temperature due to global warming Mexico, only the cannonball jellyfish Stomolo- [2,3], reduction of predators by overfishing, and water phus meleagris is captured commercially. Most pollution [4,5] have been mentioned. Waste discharge of the capture of this jellyfish species is ob- into the sea, and in general, the increment of pollution in tained within the Gulf of California, specifically in the state of Sonora. -
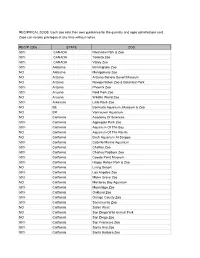
2006 Reciprocal List
RECIPRICAL ZOOS. Each zoo sets their own guidelines for the quantity and ages admitted per card. Zoos can revoke privileges at any time without notice. RECIP 2006 STATE ZOO 50% CANADA Riverview Park & Zoo 50% CANADA Toronto Zoo 50% CANADA Valley Zoo 50% Alabama Birmingham Zoo NO Alabama Montgomery Zoo NO Arizona Arizona-Sonora Desert Museum NO Arizona Navajo Nation Zoo & Botanical Park 50% Arizona Phoenix Zoo 50% Arizona Reid Park Zoo NO Arizona Wildlife World Zoo 50% Arkansas Little Rock Zoo NO BE Bermuda Aquarium, Museum & Zoo NO BR Vancouver Aquarium NO California Academy Of Sciences 50% California Applegate Park Zoo 50% California Aquarium Of The Bay NO California Aquarium Of The Pacific NO California Birch Aquarium At Scripps 50% California Cabrillo Marine Aquarium 50% California Chaffee Zoo 50% California Charles Paddock Zoo 50% California Coyote Point Museum 50% California Happy Hollow Park & Zoo NO California Living Desert 50% California Los Angeles Zoo 50% California Micke Grove Zoo NO California Monterey Bay Aquarium 50% California Moonridge Zoo 50% California Oakland Zoo 50% California Orange County Zoo 50% California Sacramento Zoo NO California Safari West NO California San Diego Wild Animal Park NO California San Diego Zoo 50% California San Francisco Zoo 50% California Santa Ana Zoo 50% California Santa Barbara Zoo NO California Seaworld San Diego 50% California Sequoia Park Zoo NO California Six Flags Marine World NO California Steinhart Aquarium NO CANADA Calgary Zoo 50% Colorado Butterfly Pavilion NO Colorado Cheyenne -
Poster Final Copy
Introduction to Jellyfish Jellyfish have been around for over 700 million years making them one of the oldest living creatures on earth. There are almost 3,000 species of jellyfish found throughout the world's oceans. From Cnidarian jellyfish, which are often referred as “true” jellyfish, to Ctenophores (comb jellyfish), Cubic Aquarium Systems have compiled this poster showing some of the most interesting species found around the globe. Moon Jellyfish Amakusa Jellyfish Mauve Stinger Purple Striped Jellyfish Japanese Sea Nettle Aurelia aurita Sanderia malayensis Pelagia noctilca Chrysaora colorata Chrysaora pacifica Pacific Sea Nettle Black Sea Nettle Lion’s Mane Jellyfish Blue Fire Jellyfish Egg Yolk Jellyfish Chrysaora fuscescens Chrysaora achlyos Cyanea capillata Cyanea lamarckii Phacellophora camtschatica Flame Jellyfish Blue Blubber Spotted Lagoon Jellyfish Australian Spotted Lagoon Jellyfish Upside Down Jellyfish Rhopilema esculentum Catostylus mosaicus Mastigias papu Phyllorhiza punctata Cassioppea sp. Mediterranean Jellyfish Crown Jellyfish Purple Jellyfish Chrystal Jellyfish Flower Hat Jellyfish Cotylorhiza tuberculata Cephea cephea Thysanostoma thysanura Aequorea victoria Olindias formosa Immortal Jellyfish Portuguese Man O’ War Box Jellyfish Comb Jellyfish Sea Gooseberry Turritopsis dohrni Physalia physali Chironex sp. Bolinopsis sp. Pleurobrachia bachei Cubic Aquarium Systems Exotic Aquaculture Jellyfish aquariums and specialised fish tanks | Cubic Aquarium Syste International Jellyfish Wholesale Cubic Aquarium Systems build a range of unique, specialised aquariums Exotic Aquaculture are a Hong Kong based aquarium livestock supplier for jellyfish and other unusual sea creatures specializing in jellyfish wholesale to the public and home aquarium trade. www.cubicaquarium.com Copyright Sanderia Group Limited www.exoticaquaculture.com All rights reserved. -

Giant Pacific Octopus (Enteroctopus Dofleini) Care Manual
Giant Pacific Octopus Insert Photo within this space (Enteroctopus dofleini) Care Manual CREATED BY AZA Aquatic Invertebrate Taxonomic Advisory Group IN ASSOCIATION WITH AZA Animal Welfare Committee Giant Pacific Octopus (Enteroctopus dofleini) Care Manual Giant Pacific Octopus (Enteroctopus dofleini) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Aquatic Invertebrate Taxon Advisory Group (AITAG) (2014). Giant Pacific Octopus (Enteroctopus dofleini) Care Manual. Association of Zoos and Aquariums, Silver Spring, MD. Original Completion Date: September 2014 Dedication: This work is dedicated to the memory of Roland C. Anderson, who passed away suddenly before its completion. No one person is more responsible for advancing and elevating the state of husbandry of this species, and we hope his lifelong body of work will inspire the next generation of aquarists towards the same ideals. Authors and Significant Contributors: Barrett L. Christie, The Dallas Zoo and Children’s Aquarium at Fair Park, AITAG Steering Committee Alan Peters, Smithsonian Institution, National Zoological Park, AITAG Steering Committee Gregory J. Barord, City University of New York, AITAG Advisor Mark J. Rehling, Cleveland Metroparks Zoo Roland C. Anderson, PhD Reviewers: Mike Brittsan, Columbus Zoo and Aquarium Paula Carlson, Dallas World Aquarium Marie Collins, Sea Life Aquarium Carlsbad David DeNardo, New York Aquarium Joshua Frey Sr., Downtown Aquarium Houston Jay Hemdal, Toledo