Clearance Rates of Jellyfish and Their Potential Predation Impact on Zooplankton and Fish Larvae in a Neritic Ecosystem (Limfjorden, Denmark)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydrozoa of the Eurasian Arctic Seas 397 S
THE ARCTIC SEAS CI imatology, Oceanography, Geology, and Biology Edited by Yvonne Herman IOm51 VAN NOSTRAND REINHOLD COMPANY ~ -----New York This work relates to Department of the Navy Grant NOOOI4-85- G-0252 issued by the Office of Naval Research. The United States Government has a royalty-free license throughout the world in all copyrightable material contained herein. Copyright © 1989 by Van Nostrand Reinhold Softcover reprint of the hardcover 1st edition 1989 Library of Congress Catalog Card Number 88-33800 ISBN-13 :978-1-4612-8022-4 e-ISBN-13: 978-1-4613-0677-1 DOI: 10.1007/978-1-4613-0677-1 All rights reserved. No part of this work covered by the copyright hereon may be reproduced or used in any form or by any means-graphic, electronic, or mechanical, including photocopying, recording, taping, or information storage and retrieval systems-without written permission of the publisher. Designed by Beehive Production Services Van Nostrand Reinhold 115 Fifth Avenue New York, New York 10003 Van Nostrand Reinhold (International) Limited 11 New Fetter Lane London EC4P 4EE, England Van Nostrand Reinhold 480 La Trobe Street Melbourne, Victoria 3000, Australia Nelson Canada 1120 Birchmount Road Scarborough, Ontario MIK 5G4, Canada 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 Library of Congress Cataloging in Publication Data The Arctic Seas. Includes index. 1. Oceanography-Arctic Ocean. 2. Geology-ArctiC Ocean. 1. Herman, Yvonne. GC401.A76 1989 551.46'8 88-33800 ISBN-13: 978-1-4612-8022-4 For Anyu Contents Preface / vii Contributors / ix 1. -

The First Record of Bougainvillia Principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) from the White Sea
Invertebrate Zoology, 2018, 15(4): 333–339 © INVERTEBRATE ZOOLOGY, 2018 The first record of Bougainvillia principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) from the White Sea A.A. Prudkovsky1, T.V. Neretina2,3 1 Dept. Invertebrate Zoology, Faculty of Biology, Lomonosov Moscow State University, Leninskie Gory 1–12, 119991 Moscow, Russia. E-mail: [email protected] 2 Pertsov White Sea Biological Station, Biological Faculty, Moscow State University M.V. Lomonos- ov, Leninskie Gory 1-12, 119991 Moscow, Russia. 3 Pirogov Russian National Research Medical University, Ostrovitianov 1, 117997 Moscow, Russia. ABSTRACT: Hydroids are common components of fouling communities in the sea, but they are often inconspicuous and easily overlooked. In such cases, the appearance of their medusae in plankton is an obvious indicator of the species’ presence in a locality. In this study, we present the first record of medusae Bougainvillia principis from the White Sea. We hypothesize that hydroids of the species B. principis inhabit the White Sea, as well, but they do not usually produce medusae and consequently the species does not exhibit sexual reproduction in the White Sea. How to cite this article: Prudkovsky A.A., Neretina T.V. 2018. The first record of Bougainvillia principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) from the White Sea // Invert. Zool. Vol.15. No.4. P. 333–339. doi: 10.15298/invertzool.15.4.02 KEY WORDS: Bougainvillia principis, medusa, first report, White Sea. Первая находка медузы Bougainvillia principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) в Белом море А.A. Прудковский1, Т.В. Неретина2,3 1 Кафедра зоологии беспозвоночных, Биологический факультет МГУ имени М.В. -
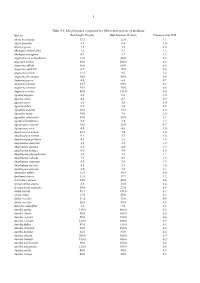
PDF (Table·S1. Morphospace Computed for 660 Extant Species Of
1 Table·S1. Morphospace computed for 660 extant species of medusae Species Bell height H (mm) Bell diameter D (mm) Fineness ratio H/D Abyla bicarinata 13.3 12.4 1.1 Abyla haeckeli 5.8 6.0 1.0 Abyla trigona 7.5 5.1 1.5 Abylopsis eschscholtzi 7.6 5.7 1.3 Abylopsis tetragona 4.9 3.7 1.3 Aeginodiscus actinodiscus 13.0 40.0 0.3 Aeginura beebei 18.0 100.0 0.2 Aequorea albida 30.0 60.0 0.5 Aequorea australis 6.7 18.0 0.4 Aequorea conica 11.0 9.0 1.2 Aequorea floridiana 14.8 40.0 0.4 Aequorea parva 4.0 6.0 0.7 Aequorea tennuis 25.7 90.0 0.3 Aequorea victoria 35.0 70.0 0.5 Aequorea vitrina 50.0 135.0 0.4 Agalma elegans 8.8 8.6 1.0 Agalma okeni 4.4 4.7 0.9 Agastra mira 1.0 1.0 1.0 Agastra rubra 0.8 1.0 0.8 Aglantha digitale 30.0 15.0 2.0 Aglantha ignea 14.0 7.0 2.0 Aglantha intermedia 15.0 14.0 1.1 Aglaura hemistoma 5.0 3.5 1.4 Aglauropsis conanti 15.0 22.0 0.7 Aglauropsis jarli 4.0 4.0 1.0 Amphicaryon acaule 10.3 7.4 1.4 Amphicaryon ernesti 9.2 5.7 1.6 Amphicaryon peltifera 4.3 2.6 1.6 Amphinema australis 3.0 2.5 1.2 Amphinema dinema 6.0 4.0 1.5 Amphinema krampi 6.0 4.0 1.5 Amphinema physophorum 2.0 1.8 1.1 Amphinema rubrum 7.0 4.5 1.6 Amphinema rugusum 5.0 3.0 1.7 Amphinema turrida 5.5 5.3 1.0 Amphogona apicata 8.0 7.0 1.1 Annatiara affinis 12.0 14.5 0.8 Apolemia uvaria 21.8 17.7 1.2 Archirhiza aurosa 14.0 40.0 0.4 Arctapodema ampla 5.6 15.0 0.4 Arctapodema australis 14.0 23.0 0.6 Atolla bairdii 46.7 144.0 0.3 Atolla chuni 15.0 50.0 0.3 Atolla wyvillei 31.5 73.0 0.4 Atolla wyvillei 26.0 55.0 0.5 Atorella vanhoffeni 3.0 7.0 0.4 Aurelia aurita -

SPECIAL PUBLICATION 6 the Effects of Marine Debris Caused by the Great Japan Tsunami of 2011
PICES SPECIAL PUBLICATION 6 The Effects of Marine Debris Caused by the Great Japan Tsunami of 2011 Editors: Cathryn Clarke Murray, Thomas W. Therriault, Hideaki Maki, and Nancy Wallace Authors: Stephen Ambagis, Rebecca Barnard, Alexander Bychkov, Deborah A. Carlton, James T. Carlton, Miguel Castrence, Andrew Chang, John W. Chapman, Anne Chung, Kristine Davidson, Ruth DiMaria, Jonathan B. Geller, Reva Gillman, Jan Hafner, Gayle I. Hansen, Takeaki Hanyuda, Stacey Havard, Hirofumi Hinata, Vanessa Hodes, Atsuhiko Isobe, Shin’ichiro Kako, Masafumi Kamachi, Tomoya Kataoka, Hisatsugu Kato, Hiroshi Kawai, Erica Keppel, Kristen Larson, Lauran Liggan, Sandra Lindstrom, Sherry Lippiatt, Katrina Lohan, Amy MacFadyen, Hideaki Maki, Michelle Marraffini, Nikolai Maximenko, Megan I. McCuller, Amber Meadows, Jessica A. Miller, Kirsten Moy, Cathryn Clarke Murray, Brian Neilson, Jocelyn C. Nelson, Katherine Newcomer, Michio Otani, Gregory M. Ruiz, Danielle Scriven, Brian P. Steves, Thomas W. Therriault, Brianna Tracy, Nancy C. Treneman, Nancy Wallace, and Taichi Yonezawa. Technical Editor: Rosalie Rutka Please cite this publication as: The views expressed in this volume are those of the participating scientists. Contributions were edited for Clarke Murray, C., Therriault, T.W., Maki, H., and Wallace, N. brevity, relevance, language, and style and any errors that [Eds.] 2019. The Effects of Marine Debris Caused by the were introduced were done so inadvertently. Great Japan Tsunami of 2011, PICES Special Publication 6, 278 pp. Published by: Project Designer: North Pacific Marine Science Organization (PICES) Lori Waters, Waters Biomedical Communications c/o Institute of Ocean Sciences Victoria, BC, Canada P.O. Box 6000, Sidney, BC, Canada V8L 4B2 Feedback: www.pices.int Comments on this volume are welcome and can be sent This publication is based on a report submitted to the via email to: [email protected] Ministry of the Environment, Government of Japan, in June 2017. -

CNIDARIA Corals, Medusae, Hydroids, Myxozoans
FOUR Phylum CNIDARIA corals, medusae, hydroids, myxozoans STEPHEN D. CAIRNS, LISA-ANN GERSHWIN, FRED J. BROOK, PHILIP PUGH, ELLIOT W. Dawson, OscaR OcaÑA V., WILLEM VERvooRT, GARY WILLIAMS, JEANETTE E. Watson, DENNIS M. OPREsko, PETER SCHUCHERT, P. MICHAEL HINE, DENNIS P. GORDON, HAMISH J. CAMPBELL, ANTHONY J. WRIGHT, JUAN A. SÁNCHEZ, DAPHNE G. FAUTIN his ancient phylum of mostly marine organisms is best known for its contribution to geomorphological features, forming thousands of square Tkilometres of coral reefs in warm tropical waters. Their fossil remains contribute to some limestones. Cnidarians are also significant components of the plankton, where large medusae – popularly called jellyfish – and colonial forms like Portuguese man-of-war and stringy siphonophores prey on other organisms including small fish. Some of these species are justly feared by humans for their stings, which in some cases can be fatal. Certainly, most New Zealanders will have encountered cnidarians when rambling along beaches and fossicking in rock pools where sea anemones and diminutive bushy hydroids abound. In New Zealand’s fiords and in deeper water on seamounts, black corals and branching gorgonians can form veritable trees five metres high or more. In contrast, inland inhabitants of continental landmasses who have never, or rarely, seen an ocean or visited a seashore can hardly be impressed with the Cnidaria as a phylum – freshwater cnidarians are relatively few, restricted to tiny hydras, the branching hydroid Cordylophora, and rare medusae. Worldwide, there are about 10,000 described species, with perhaps half as many again undescribed. All cnidarians have nettle cells known as nematocysts (or cnidae – from the Greek, knide, a nettle), extraordinarily complex structures that are effectively invaginated coiled tubes within a cell. -

The C4 Plant Lineages of Planet Earth
Journal of Experimental Botany, Vol. 62, No. 9, pp. 3155–3169, 2011 doi:10.1093/jxb/err048 Advance Access publication 16 March, 2011 REVIEW PAPER The C4 plant lineages of planet Earth Rowan F. Sage1,*, Pascal-Antoine Christin2 and Erika J. Edwards2 1 Department of Ecology and Evolutionary Biology, The University of Toronto, 25 Willcocks Street, Toronto, Ontario M5S3B2 Canada 2 Department of Ecology and Evolutionary Biology, Brown University, 80 Waterman St., Providence, RI 02912, USA * To whom correspondence should be addressed. E-mail: [email protected] Received 30 November 2010; Revised 1 February 2011; Accepted 2 February 2011 Abstract Using isotopic screens, phylogenetic assessments, and 45 years of physiological data, it is now possible to identify most of the evolutionary lineages expressing the C4 photosynthetic pathway. Here, 62 recognizable lineages of C4 photosynthesis are listed. Thirty-six lineages (60%) occur in the eudicots. Monocots account for 26 lineages, with a Downloaded from minimum of 18 lineages being present in the grass family and six in the sedge family. Species exhibiting the C3–C4 intermediate type of photosynthesis correspond to 21 lineages. Of these, 9 are not immediately associated with any C4 lineage, indicating that they did not share common C3–C4 ancestors with C4 species and are instead an independent line. The geographic centre of origin for 47 of the lineages could be estimated. These centres tend to jxb.oxfordjournals.org cluster in areas corresponding to what are now arid to semi-arid regions of southwestern North America, south- central South America, central Asia, northeastern and southern Africa, and inland Australia. -

Hydroids and Hydromedusae of Southern Chesapeake Bay
W&M ScholarWorks Reports 1971 Hydroids and hydromedusae of southern Chesapeake Bay Dale Calder Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/reports Part of the Marine Biology Commons, Oceanography Commons, Terrestrial and Aquatic Ecology Commons, and the Zoology Commons Recommended Citation Calder, D. (1971) Hydroids and hydromedusae of southern Chesapeake Bay. Special papers in marine science; No. 1.. Virginia Institute of Marine Science, William & Mary. http://doi.org/10.21220/V5MS31 This Report is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in Reports by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. LIST OF TABLES Table Page Data on Moerisia lyonsi medusae ginia ...................... 21 rugosa medusae 37 Comparison of hydroids from Virginia, with colonies from Passamaquoddy Bay, New Brunswick.. .................. Hydroids reported from the Virginia Institute of Marine Science (Virginia Fisheries Laboratory) collection up to 1959 ................................................ Zoogeographical comparisons of the hydroid fauna along the eastern United States ............................... List of hydroids from Chesapeake Bay, with their east coast distribution ...me..................................O 8. List of hydromedusae known from ~hesa~eakeBay and their east coast distribution .................................. LIST OF FIGURES Figure Page 1. Southern Chesapeake Bay and adjacent water^.............^^^^^^^^^^^^^^^^^^^^^^^^ 2. Oral view of Maeotias inexpectata ........e~~~~~e~~~~~~a~~~~~~~~~~~o~~~~~~e 3. rature at Gloucester Point, 1966-1967..........a~e.ee~e~~~~~~~aeaeeeeee~e 4. Salinity at Gloucester Point, 1966-1967..........se0me~BIBIeBIBI.e.BIBIBI.BIBIBIs~e~eeemeea~ LIST OF PLATES Plate Hydroids, Moerisia lyonsi to Cordylophora caspia a a e..a a * a 111 ................... -

Uchida, Tohru Cita
A NEW HYDROID SPECIES OF CYTAEIS, WITH SOME Title REMARKS ON THE INTERRELATIONSHIPS IN THE FILIFERA Author(s) Uchida, Tohru PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1964), 12(2): 133-144 Issue Date 1964-10-15 URL http://hdl.handle.net/2433/175362 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University A NEW HYDROID SPECIES OF CYTAEIS, WITH SOME REMARKS ON THE INTERRELATIONSHIPS IN THE I<'ILIFERA ToHRU UCHIDA Zoological Institute, Faculty of Science Hokkaido University With 8 Text-figures In 1927 the writer published a paper on Japanese Anthomedusae, and herein described Cytaeis japonica as a new species on the basis of specimens collected from Misaki and Seto. In this paper he reported young medusae with medusa-buds, which was identified with a query at that time with C. japonica (in lines 20 and 38-40 on page 216), and further alluded to the identi· fication of Cytaeis vulgaris by MAAS (1909) from Japan with these young medusae. Next year upon receiving a paper from KRAMP (1928) describing a new medusa, Podocoryne simplex from Japan, the present writer learned that the young medusae with medusa-buds were not young forms of Cytaeis japonica but nothing but Podocoryne simplex. In 1930 the writer examined MAAs' specimens which were collected in Japan and preserved in the Zoologische Sammlung in Munich and made clear that the young medusae with medusa-buds are precisely Podocoryne simplex. Besides, he could find no specimen of the genus Cytaeis in this collection. Since then he reported Podocoryne simplex from several localities, from Asamushi to Amakusa, in Japan (UcHIDA, 1938 & 1938a). -

Proceedings of National Seminar on Biodiversity And
BIODIVERSITY AND CONSERVATION OF COASTAL AND MARINE ECOSYSTEMS OF INDIA (2012) --------------------------------------------------------------------------------------------------------------------------------------------------------- Patrons: 1. Hindi VidyaPracharSamiti, Ghatkopar, Mumbai 2. Bombay Natural History Society (BNHS) 3. Association of Teachers in Biological Sciences (ATBS) 4. International Union for Conservation of Nature and Natural Resources (IUCN) 5. Mangroves for the Future (MFF) Advisory Committee for the Conference 1. Dr. S. M. Karmarkar, President, ATBS and Hon. Dir., C B Patel Research Institute, Mumbai 2. Dr. Sharad Chaphekar, Prof. Emeritus, Univ. of Mumbai 3. Dr. Asad Rehmani, Director, BNHS, Mumbi 4. Dr. A. M. Bhagwat, Director, C B Patel Research Centre, Mumbai 5. Dr. Naresh Chandra, Pro-V. C., University of Mumbai 6. Dr. R. S. Hande. Director, BCUD, University of Mumbai 7. Dr. Madhuri Pejaver, Dean, Faculty of Science, University of Mumbai 8. Dr. Vinay Deshmukh, Sr. Scientist, CMFRI, Mumbai 9. Dr. Vinayak Dalvie, Chairman, BoS in Zoology, University of Mumbai 10. Dr. Sasikumar Menon, Dy. Dir., Therapeutic Drug Monitoring Centre, Mumbai 11. Dr, Sanjay Deshmukh, Head, Dept. of Life Sciences, University of Mumbai 12. Dr. S. T. Ingale, Vice-Principal, R. J. College, Ghatkopar 13. Dr. Rekha Vartak, Head, Biology Cell, HBCSE, Mumbai 14. Dr. S. S. Barve, Head, Dept. of Botany, Vaze College, Mumbai 15. Dr. Satish Bhalerao, Head, Dept. of Botany, Wilson College Organizing Committee 1. Convenor- Dr. Usha Mukundan, Principal, R. J. College 2. Co-convenor- Deepak Apte, Dy. Director, BNHS 3. Organizing Secretary- Dr. Purushottam Kale, Head, Dept. of Zoology, R. J. College 4. Treasurer- Prof. Pravin Nayak 5. Members- Dr. S. T. Ingale Dr. Himanshu Dawda Dr. Mrinalini Date Dr. -

Invertebrate Experiments and Research Projects
Invertebrate Experiments And Research Projects Charles K. Biernbaum Professor Emeritus College of Charleston This is an assemblage of exercises, experiments, and research projects that one can do utilizing invertebrates. I developed several of them while working with students; many others have come from a variety of published and unpublished sources. Although some specifically mention species found along the southeastern coast of the United States, similar species can be found elsewhere Enjoy! Protistans 1. Selective vital staining: Such staining will kill the organisms eventually, but makes detailed analysis of protistans easier. These dyes can be added as 0.01% aqueous solutions. Useful stains and what they color are as follows: Neutral red - food vacuoles, Janus green B - mitochondria, Sudan black B and Sudan IV - lipids, Brilliant cresyl blue - several structures. Although the organisms will be killed rapidly, acidified methyl green can be used to stain nuclei. If appropriate microscopes are available, phase contrast and dark field optics will provide excellent views of unstained specimens. 2. Digestion in Paramecium: Add equal amounts of Paramecium suspension and a congo red-yeast cell mixture to a wet mount. Ring the drop with vaseline to decrease desiccation and add a cover slip. Observe ingestion of the stained yeast cells by the Paramecium. Record the color of food vacuole contents after 1, 5, 10, 20, and 30 minutes. What is the significance of the color changes in the food vacuoles (orange above pH of 5; blue below pH of 3)? The congo red-yeast mixture is made by mixing 1-2g of dry yeast with 30 mg congo red and boiling for 10 minutes. -

On a Collection of Hydroids (Cnidaria, Hydrozoa) from the Southwest Coast of Florida, USA
Zootaxa 4689 (1): 001–141 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Monograph ZOOTAXA Copyright © 2019 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4689.1.1 http://zoobank.org/urn:lsid:zoobank.org:act:4C926BE2-D75D-449A-9EAD-14CADACFFADD ZOOTAXA 4689 On a collection of hydroids (Cnidaria, Hydrozoa) from the southwest coast of Florida, USA DALE R. CALDER1, 2 1Department of Natural History, Royal Ontario Museum, 100 Queen’s Park, Toronto, Ontario, Canada M5S 2C6 E-mail: [email protected] 2Research Associate, Royal British Columbia Museum, 675 Belleville Street, Victoria, British Columbia, Canada V8W 9W2. Magnolia Press Auckland, New Zealand Accepted by B. Bentlage: 9 Sept.. 2019; published: 25 Oct. 2019 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 DALE R. CALDER On a collection of hydroids (Cnidaria, Hydrozoa) from the southwest coast of Florida, USA (Zootaxa 4689) 141 pp.; 30 cm. 25 Oct. 2019 ISBN 978-1-77670-799-7 (paperback) ISBN 978-1-77670-800-0 (Online edition) FIRST PUBLISHED IN 2019 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] https://www.mapress.com/j/zt © 2019 Magnolia Press ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) 2 · Zootaxa 4689 (1) © 2019 Magnolia Press CALDER Table of Contents Abstract ...................................................................................................5 Introduction ................................................................................................5 -

Habitat Type Drives the Distribution of Non-Indigenous Species in Fouling
Habitat type drives the distribution of non-indigenous species in fouling communities regardless of associated maritime traffic Jean-charles Leclerc, Frédérique Viard, Elizabeth González Sepúlveda, Christian Díaz, José Neira Hinojosa, Karla Pérez Araneda, Francisco Silva, Antonio Brante To cite this version: Jean-charles Leclerc, Frédérique Viard, Elizabeth González Sepúlveda, Christian Díaz, José Neira Hinojosa, et al.. Habitat type drives the distribution of non-indigenous species in fouling communities regardless of associated maritime traffic. Diversity and Distributions, Wiley, 2019, 00, pp.1 -14. 10.1111/ddi.12997. hal-02372571 HAL Id: hal-02372571 https://hal.archives-ouvertes.fr/hal-02372571 Submitted on 20 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Received: 19 July 2019 | Revised: 23 September 2019 | Accepted: 28 September 2019 DOI: 10.1111/ddi.12997 BIODIVERSITY RESEARCH Habitat type drives the distribution of non‐indigenous species in fouling communities regardless of associated maritime traffic Jean‐Charles Leclerc1 | Frédérique Viard2 | Elizabeth González Sepúlveda3 | Christian Díaz4 | José Neira Hinojosa5 | Karla Pérez Araneda1 | Francisco Silva1 | Antonio Brante1 1Departamento de Ecología, Facultad de Ciencias, Centro de Investigación en Abstract Biodiversidad y Ambientes Sustentables Aim: Biological invasions and changes in land and sea use are among the five major (CIBAS), Universidad Católica de la Santísima Concepción, Concepción, Chile causes of global biodiversity decline.