Habitat Type Drives the Distribution of Non-Indigenous Species in Fouling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydrozoa of the Eurasian Arctic Seas 397 S
THE ARCTIC SEAS CI imatology, Oceanography, Geology, and Biology Edited by Yvonne Herman IOm51 VAN NOSTRAND REINHOLD COMPANY ~ -----New York This work relates to Department of the Navy Grant NOOOI4-85- G-0252 issued by the Office of Naval Research. The United States Government has a royalty-free license throughout the world in all copyrightable material contained herein. Copyright © 1989 by Van Nostrand Reinhold Softcover reprint of the hardcover 1st edition 1989 Library of Congress Catalog Card Number 88-33800 ISBN-13 :978-1-4612-8022-4 e-ISBN-13: 978-1-4613-0677-1 DOI: 10.1007/978-1-4613-0677-1 All rights reserved. No part of this work covered by the copyright hereon may be reproduced or used in any form or by any means-graphic, electronic, or mechanical, including photocopying, recording, taping, or information storage and retrieval systems-without written permission of the publisher. Designed by Beehive Production Services Van Nostrand Reinhold 115 Fifth Avenue New York, New York 10003 Van Nostrand Reinhold (International) Limited 11 New Fetter Lane London EC4P 4EE, England Van Nostrand Reinhold 480 La Trobe Street Melbourne, Victoria 3000, Australia Nelson Canada 1120 Birchmount Road Scarborough, Ontario MIK 5G4, Canada 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 Library of Congress Cataloging in Publication Data The Arctic Seas. Includes index. 1. Oceanography-Arctic Ocean. 2. Geology-ArctiC Ocean. 1. Herman, Yvonne. GC401.A76 1989 551.46'8 88-33800 ISBN-13: 978-1-4612-8022-4 For Anyu Contents Preface / vii Contributors / ix 1. -

Clearance Rates of Jellyfish and Their Potential Predation Impact on Zooplankton and Fish Larvae in a Neritic Ecosystem (Limfjorden, Denmark)
MARINE ECOLOGY PROGRESS SERIES Vol. 304: 117–131, 2005 Published December 8 Mar Ecol Prog Ser Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden, Denmark) Lars Johan Hansson1,*, Ole Moeslund2, Thomas Kiørboe1, Hans Ulrik Riisgård2 1Danish Institute for Fisheries Research, Kavalergården 6, 2920 Charlottenlund, Denmark 2Marine Biological Research Centre (University of Southern Denmark) Hindsholmvej 11, 5300 Kerteminde, Denmark ABSTRACT: Clearance rates of the hydromedusae Sarsia tubulosa, Rathkea octopunctata and Bougainvillea superciliaris and the scyphomedusa Aurelia aurita were measured in the laboratory. Gut contents analyses of A. aurita were also collected in situ and subsequently used for estimation of clearance rate. The clearance rate of A. aurita varied widely with prey organisms. Large crustacean prey with low escape capabilities (Artemia salina nauplii and cirripede larvae) were cleared at high rates, whereas copepodites were cleared at lower rates, and clearance rates of small bivalve larvae and copepod nauplii were comparatively low. These data were used to assess the impact of jellyfish preda- tion upon zooplankton and fish larvae in Limfjorden, Denmark. Repeated sampling of zooplankton, fish larvae and medusae was undertaken during the first half of 2003. Nine taxa of hydromedusae and 4 taxa of scyphomedusae were identified. Abundance estimates were combined with estimated clear- ance rates of individual medusae to calculate potential jellyfish-induced mortality on prey in Limfjor- den. Copepoda was used as a model prey group to estimate the collective predation impact by all medusae. Medusa species with unknown clearance potential were given assumed clearance rate val- ues, but the collective predation potential by these species was evaluated to be small. -

The Plankton Lifeform Extraction Tool: a Digital Tool to Increase The
Discussions https://doi.org/10.5194/essd-2021-171 Earth System Preprint. Discussion started: 21 July 2021 Science c Author(s) 2021. CC BY 4.0 License. Open Access Open Data The Plankton Lifeform Extraction Tool: A digital tool to increase the discoverability and usability of plankton time-series data Clare Ostle1*, Kevin Paxman1, Carolyn A. Graves2, Mathew Arnold1, Felipe Artigas3, Angus Atkinson4, Anaïs Aubert5, Malcolm Baptie6, Beth Bear7, Jacob Bedford8, Michael Best9, Eileen 5 Bresnan10, Rachel Brittain1, Derek Broughton1, Alexandre Budria5,11, Kathryn Cook12, Michelle Devlin7, George Graham1, Nick Halliday1, Pierre Hélaouët1, Marie Johansen13, David G. Johns1, Dan Lear1, Margarita Machairopoulou10, April McKinney14, Adam Mellor14, Alex Milligan7, Sophie Pitois7, Isabelle Rombouts5, Cordula Scherer15, Paul Tett16, Claire Widdicombe4, and Abigail McQuatters-Gollop8 1 10 The Marine Biological Association (MBA), The Laboratory, Citadel Hill, Plymouth, PL1 2PB, UK. 2 Centre for Environment Fisheries and Aquacu∑lture Science (Cefas), Weymouth, UK. 3 Université du Littoral Côte d’Opale, Université de Lille, CNRS UMR 8187 LOG, Laboratoire d’Océanologie et de Géosciences, Wimereux, France. 4 Plymouth Marine Laboratory, Prospect Place, Plymouth, PL1 3DH, UK. 5 15 Muséum National d’Histoire Naturelle (MNHN), CRESCO, 38 UMS Patrinat, Dinard, France. 6 Scottish Environment Protection Agency, Angus Smith Building, Maxim 6, Parklands Avenue, Eurocentral, Holytown, North Lanarkshire ML1 4WQ, UK. 7 Centre for Environment Fisheries and Aquaculture Science (Cefas), Lowestoft, UK. 8 Marine Conservation Research Group, University of Plymouth, Drake Circus, Plymouth, PL4 8AA, UK. 9 20 The Environment Agency, Kingfisher House, Goldhay Way, Peterborough, PE4 6HL, UK. 10 Marine Scotland Science, Marine Laboratory, 375 Victoria Road, Aberdeen, AB11 9DB, UK. -

List of Marine Alien and Invasive Species
Table 1: The list of 96 marine alien and invasive species recorded along the coastline of South Africa. Phylum Class Taxon Status Common name Natural Range ANNELIDA Polychaeta Alitta succinea Invasive pile worm or clam worm Atlantic coast ANNELIDA Polychaeta Boccardia proboscidea Invasive Shell worm Northern Pacific ANNELIDA Polychaeta Dodecaceria fewkesi Alien Black coral worm Pacific Northern America ANNELIDA Polychaeta Ficopomatus enigmaticus Invasive Estuarine tubeworm Australia ANNELIDA Polychaeta Janua pagenstecheri Alien N/A Europe ANNELIDA Polychaeta Neodexiospira brasiliensis Invasive A tubeworm West Indies, Brazil ANNELIDA Polychaeta Polydora websteri Alien oyster mudworm N/A ANNELIDA Polychaeta Polydora hoplura Invasive Mud worm Europe, Mediterranean ANNELIDA Polychaeta Simplaria pseudomilitaris Alien N/A Europe BRACHIOPODA Lingulata Discinisca tenuis Invasive Disc lamp shell Namibian Coast BRYOZOA Gymnolaemata Virididentula dentata Invasive Blue dentate moss animal Indo-Pacific BRYOZOA Gymnolaemata Bugulina flabellata Invasive N/A N/A BRYOZOA Gymnolaemata Bugula neritina Invasive Purple dentate mos animal N/A BRYOZOA Gymnolaemata Conopeum seurati Invasive N/A Europe BRYOZOA Gymnolaemata Cryptosula pallasiana Invasive N/A Europe BRYOZOA Gymnolaemata Watersipora subtorquata Invasive Red-rust bryozoan Caribbean CHLOROPHYTA Ulvophyceae Cladophora prolifera Invasive N/A N/A CHLOROPHYTA Ulvophyceae Codium fragile Invasive green sea fingers Korea CHORDATA Actinopterygii Cyprinus carpio Invasive Common carp Asia CHORDATA Ascidiacea -

The First Record of Bougainvillia Principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) from the White Sea
Invertebrate Zoology, 2018, 15(4): 333–339 © INVERTEBRATE ZOOLOGY, 2018 The first record of Bougainvillia principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) from the White Sea A.A. Prudkovsky1, T.V. Neretina2,3 1 Dept. Invertebrate Zoology, Faculty of Biology, Lomonosov Moscow State University, Leninskie Gory 1–12, 119991 Moscow, Russia. E-mail: [email protected] 2 Pertsov White Sea Biological Station, Biological Faculty, Moscow State University M.V. Lomonos- ov, Leninskie Gory 1-12, 119991 Moscow, Russia. 3 Pirogov Russian National Research Medical University, Ostrovitianov 1, 117997 Moscow, Russia. ABSTRACT: Hydroids are common components of fouling communities in the sea, but they are often inconspicuous and easily overlooked. In such cases, the appearance of their medusae in plankton is an obvious indicator of the species’ presence in a locality. In this study, we present the first record of medusae Bougainvillia principis from the White Sea. We hypothesize that hydroids of the species B. principis inhabit the White Sea, as well, but they do not usually produce medusae and consequently the species does not exhibit sexual reproduction in the White Sea. How to cite this article: Prudkovsky A.A., Neretina T.V. 2018. The first record of Bougainvillia principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) from the White Sea // Invert. Zool. Vol.15. No.4. P. 333–339. doi: 10.15298/invertzool.15.4.02 KEY WORDS: Bougainvillia principis, medusa, first report, White Sea. Первая находка медузы Bougainvillia principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) в Белом море А.A. Прудковский1, Т.В. Неретина2,3 1 Кафедра зоологии беспозвоночных, Биологический факультет МГУ имени М.В. -
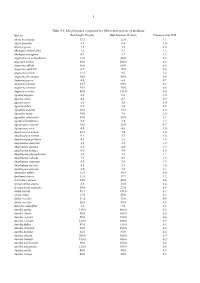
PDF (Table·S1. Morphospace Computed for 660 Extant Species Of
1 Table·S1. Morphospace computed for 660 extant species of medusae Species Bell height H (mm) Bell diameter D (mm) Fineness ratio H/D Abyla bicarinata 13.3 12.4 1.1 Abyla haeckeli 5.8 6.0 1.0 Abyla trigona 7.5 5.1 1.5 Abylopsis eschscholtzi 7.6 5.7 1.3 Abylopsis tetragona 4.9 3.7 1.3 Aeginodiscus actinodiscus 13.0 40.0 0.3 Aeginura beebei 18.0 100.0 0.2 Aequorea albida 30.0 60.0 0.5 Aequorea australis 6.7 18.0 0.4 Aequorea conica 11.0 9.0 1.2 Aequorea floridiana 14.8 40.0 0.4 Aequorea parva 4.0 6.0 0.7 Aequorea tennuis 25.7 90.0 0.3 Aequorea victoria 35.0 70.0 0.5 Aequorea vitrina 50.0 135.0 0.4 Agalma elegans 8.8 8.6 1.0 Agalma okeni 4.4 4.7 0.9 Agastra mira 1.0 1.0 1.0 Agastra rubra 0.8 1.0 0.8 Aglantha digitale 30.0 15.0 2.0 Aglantha ignea 14.0 7.0 2.0 Aglantha intermedia 15.0 14.0 1.1 Aglaura hemistoma 5.0 3.5 1.4 Aglauropsis conanti 15.0 22.0 0.7 Aglauropsis jarli 4.0 4.0 1.0 Amphicaryon acaule 10.3 7.4 1.4 Amphicaryon ernesti 9.2 5.7 1.6 Amphicaryon peltifera 4.3 2.6 1.6 Amphinema australis 3.0 2.5 1.2 Amphinema dinema 6.0 4.0 1.5 Amphinema krampi 6.0 4.0 1.5 Amphinema physophorum 2.0 1.8 1.1 Amphinema rubrum 7.0 4.5 1.6 Amphinema rugusum 5.0 3.0 1.7 Amphinema turrida 5.5 5.3 1.0 Amphogona apicata 8.0 7.0 1.1 Annatiara affinis 12.0 14.5 0.8 Apolemia uvaria 21.8 17.7 1.2 Archirhiza aurosa 14.0 40.0 0.4 Arctapodema ampla 5.6 15.0 0.4 Arctapodema australis 14.0 23.0 0.6 Atolla bairdii 46.7 144.0 0.3 Atolla chuni 15.0 50.0 0.3 Atolla wyvillei 31.5 73.0 0.4 Atolla wyvillei 26.0 55.0 0.5 Atorella vanhoffeni 3.0 7.0 0.4 Aurelia aurita -

(Allman, 1859). Sarsia Eximia Es Una Especie De Hidroide Atecado
Método de Evaluación Rápida de Invasividad (MERI) para especies exóticas en México Sarsia eximia (Allman, 1859). Sarsia eximia (Allman, 1859). Foto (c) Ken-ichi Ueda, algunos derechos reservados (CC BY-NC-SA) (http://www.naturalista.mx/taxa/51651-Sarsia-eximia) Sarsia eximia es una especie de hidroide atecado perteneciente a la familia Corynidae. Su distribución geográfica es muy amplia, siendo registrada en localidades costeras de todo el mundo. Se puede encontrar en una amplia gama de hábitats de la costa rocosa, pero también es abundante en algas y puede a menudo ser encontrado en las cuerdas y flotadores de trampas para langostas (GBIF, 2016). Información taxonómica Reino: Animalia Phylum: Cnidaria Clase: Hydrozoa Orden: Anthoathecata Familia: Corynidae Género: Sarsia Especie: Sarsia eximia (Allman, 1859) Nombre común: coral de fuego Sinónimo: Coryne eximia. Resultado: 0.2469 Categoría de riesgo: Medio Método de Evaluación Rápida de Invasividad (MERI) para especies exóticas en México Sarsia eximia (Allman, 1859). Descripción de la especie Sarsia eximia es una especie de hidroide marino colonial, formado por una hidrorriza filiforme de 184 micras de diámetro que recorre el substrato y de la que se elevan hidrocaules monosifónicos, que pueden alcanzar 2.3 cm de altura, con ramificaciones frecuentes, y ramas en un sólo plano, orientación distal y generalmente con un recurbamiento basal. Su diámetro es bastante uniforme en toda su longitud, con un perisarco espeso, liso u ondulado, y con numerosas anillaciones espaciadas. Típicamente se presentan 11-20 basales en el hidrocaule, y un número similar o superior en el inicio de las ramas, aunque asimismo existen algunas pequeñas agrupaciones irregulares en diversos puntos de la colonia, y en la totalidad de algunas ramas. -

SPECIAL PUBLICATION 6 the Effects of Marine Debris Caused by the Great Japan Tsunami of 2011
PICES SPECIAL PUBLICATION 6 The Effects of Marine Debris Caused by the Great Japan Tsunami of 2011 Editors: Cathryn Clarke Murray, Thomas W. Therriault, Hideaki Maki, and Nancy Wallace Authors: Stephen Ambagis, Rebecca Barnard, Alexander Bychkov, Deborah A. Carlton, James T. Carlton, Miguel Castrence, Andrew Chang, John W. Chapman, Anne Chung, Kristine Davidson, Ruth DiMaria, Jonathan B. Geller, Reva Gillman, Jan Hafner, Gayle I. Hansen, Takeaki Hanyuda, Stacey Havard, Hirofumi Hinata, Vanessa Hodes, Atsuhiko Isobe, Shin’ichiro Kako, Masafumi Kamachi, Tomoya Kataoka, Hisatsugu Kato, Hiroshi Kawai, Erica Keppel, Kristen Larson, Lauran Liggan, Sandra Lindstrom, Sherry Lippiatt, Katrina Lohan, Amy MacFadyen, Hideaki Maki, Michelle Marraffini, Nikolai Maximenko, Megan I. McCuller, Amber Meadows, Jessica A. Miller, Kirsten Moy, Cathryn Clarke Murray, Brian Neilson, Jocelyn C. Nelson, Katherine Newcomer, Michio Otani, Gregory M. Ruiz, Danielle Scriven, Brian P. Steves, Thomas W. Therriault, Brianna Tracy, Nancy C. Treneman, Nancy Wallace, and Taichi Yonezawa. Technical Editor: Rosalie Rutka Please cite this publication as: The views expressed in this volume are those of the participating scientists. Contributions were edited for Clarke Murray, C., Therriault, T.W., Maki, H., and Wallace, N. brevity, relevance, language, and style and any errors that [Eds.] 2019. The Effects of Marine Debris Caused by the were introduced were done so inadvertently. Great Japan Tsunami of 2011, PICES Special Publication 6, 278 pp. Published by: Project Designer: North Pacific Marine Science Organization (PICES) Lori Waters, Waters Biomedical Communications c/o Institute of Ocean Sciences Victoria, BC, Canada P.O. Box 6000, Sidney, BC, Canada V8L 4B2 Feedback: www.pices.int Comments on this volume are welcome and can be sent This publication is based on a report submitted to the via email to: [email protected] Ministry of the Environment, Government of Japan, in June 2017. -

CNIDARIA Corals, Medusae, Hydroids, Myxozoans
FOUR Phylum CNIDARIA corals, medusae, hydroids, myxozoans STEPHEN D. CAIRNS, LISA-ANN GERSHWIN, FRED J. BROOK, PHILIP PUGH, ELLIOT W. Dawson, OscaR OcaÑA V., WILLEM VERvooRT, GARY WILLIAMS, JEANETTE E. Watson, DENNIS M. OPREsko, PETER SCHUCHERT, P. MICHAEL HINE, DENNIS P. GORDON, HAMISH J. CAMPBELL, ANTHONY J. WRIGHT, JUAN A. SÁNCHEZ, DAPHNE G. FAUTIN his ancient phylum of mostly marine organisms is best known for its contribution to geomorphological features, forming thousands of square Tkilometres of coral reefs in warm tropical waters. Their fossil remains contribute to some limestones. Cnidarians are also significant components of the plankton, where large medusae – popularly called jellyfish – and colonial forms like Portuguese man-of-war and stringy siphonophores prey on other organisms including small fish. Some of these species are justly feared by humans for their stings, which in some cases can be fatal. Certainly, most New Zealanders will have encountered cnidarians when rambling along beaches and fossicking in rock pools where sea anemones and diminutive bushy hydroids abound. In New Zealand’s fiords and in deeper water on seamounts, black corals and branching gorgonians can form veritable trees five metres high or more. In contrast, inland inhabitants of continental landmasses who have never, or rarely, seen an ocean or visited a seashore can hardly be impressed with the Cnidaria as a phylum – freshwater cnidarians are relatively few, restricted to tiny hydras, the branching hydroid Cordylophora, and rare medusae. Worldwide, there are about 10,000 described species, with perhaps half as many again undescribed. All cnidarians have nettle cells known as nematocysts (or cnidae – from the Greek, knide, a nettle), extraordinarily complex structures that are effectively invaginated coiled tubes within a cell. -

Coryne Eximia Sottoclasse Anthomedusae Allman, 1859 Ordine Capitata Famiglia Corynidae
Identificazione e distribuzione nei mari italiani di specie non indigene Classe Hydroidomedusa Coryne eximia Sottoclasse Anthomedusae Allman, 1859 Ordine Capitata Famiglia Corynidae SINONIMI RILEVANTI Coryne tenella Syncoryne eximia Syncoryne tenella DESCRIZIONE COROLOGIA / AFFINITA’ Idrante con 4-5 tentacoli orali capitati disposti in Senza dati. un giro e 25-30 tentacoli capitati sparsi o disposti in giri lungo l'idrante; i gonozoidi originano meduse. DISTRIBUZIONE ATTUALE Medusa adulta alta circa 2-3 mm, a forma di Atlantico, Indo-Pacifico, Artico, Mediterraneo. campana; 4 canali radiali; bulbi provvisti di camera gastrodermale arrotondata rossa un ocello PRIMA SEGNALAZIONE IN MEDITERRANEO rosso su ogni bulbo marginale; manubrio Marsiglia e Villefranche-sur-Mer - Kramp 1957 cilindrico. (solo stadio di medusa). (Le segnalazioni precedenti Cnidoma: stenotele di due taglie nei polipi, sono dubbie). stenotele e desmoneme nelle meduse. COLORAZIONE PRIMA SEGNALAZIONE IN ITALIA Idranti arancio-rossi, alcuni verdastri. Ocelli delle Portofino - Puce et al. 2003 (primo record dello meduse marroni-rossastri scuri; manubrio stadio di polipo). verdastro. ORIGINE FORMULA MERISTICA Non è possibile determinarla. - VIE DI DISPERSIONE PRIMARIE TAGLIA MASSIMA Sconosciute. - STADI LARVALI VIE DI DISPERSIONE SECONDARIE - - Identificazione e distribuzione nei mari italiani di specie non indigene SPECIE SIMILI STATO DELL ’INVASIONE Specie invasiva. - MOTIVI DEL SUCCESSO CARATTERI DISTINTIVI Sconosciuti. - SPECIE IN COMPETIZIONE - HABITAT IMPATTI Polipo bentonico, medusa planctonica. - PARTICOLARI CONDIZIONI AMBIENTALI DANNI ECOLOGICI Sconosciute. - DANNI ECONOMICI BIOLOGIA - Sconosciuta. IMPORTANZA PER L ’UOMO Sconosciuta. BANCA DEI CAMPIONI - PRESENZA IN G -BANK - PROVENIENZA DEL CAMPIONE TIPOLOGIA : (MUSCOLO / ESEMPLARE INTERO / CONGELATO / FISSATO ECC ) LUOGO DI CONSERVAZIONE CODICE CAMPIONE Identificazione e distribuzione nei mari italiani di specie non indigene BIBLIOGRAFIA Kramp P.L., 1957. -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department. -

Cnidaria) Estuarinos Do Sudeste E Sul Do Brasil
Juliana Bardi Comunidades de hidrozoários (Cnidaria) estuarinos do sudeste e sul do Brasil São Paulo 2011 Juliana Bardi Comunidades de hidrozoários (Cnidaria) estuarinos do sudeste e sul do Brasil Tese apresentada ao Instituto de Biociências da Universidade de São Paulo, para obtenção do Título de Doutor em Ciências, na área de Zoologia. Orientador: Antonio Carlos Marques São Paulo 2011 Bardi, J. Comunidades de hidrozoários (Cnidaria) estuarinos do sudeste e sul do Brasil. 188 páginas Tese (Doutorado) – Instituto de Biociências da Universidade de São Paulo. Departamento de Zoologia. 1. Hydrozoa 2. Estuários 3. Salinidade 4. Sazonalidade 5. Espécies invasoras I. Universidade de São Paulo. Instituto de Biociências. Departamento de Zoologia Comissão Julgadora __________________________ __________________________ Prof.(a) Dr.(a): Prof.(a) Dr.(a): __________________________ __________________________ Prof.(a) Dr.(a): Prof.(a) Dr.(a): __________________________ Prof. Dr. Antonio Carlos Marques Orientador “And for every mystery solved, a dozen more present themselves.” (Fabien Cousteau) Aos meus queridos pais, com todo meu amor. AGRADECIMENTOS Ao meu orientador, Antonio Carlos Marques (Tim), pela oportunidade, orientação, ensinamentos e pela paciência ao longo desses anos. Ao Departamento de Zoologia do Instituto de Biociências da USP e a todos os professores e funcionários, por viabilizarem a execução deste estudo. Em especial a Ênio Mattos pela constante dedicação e disponibilidade em ajudar. Ao Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) pelos cinco primeiros meses de bolsa de doutorado. À Fundação de Amparo à Pesquisa do estado de São Paulo, FAPESP pela concessão da bolsa de doutorado. Ao Prof. Dr. Mário Katsuragawa (IO-USP) e à Lourdes Zani Teixeira (IO-USP) pela gentileza no empréstimo de equipamentos para coleta (fluxômetro e draga Van-Veem).