Gateways of Ventral and Dorsal Streams in Mouse Visual Cortex Quanxin Wang Washington University School of Medicine in St
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
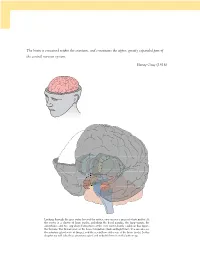
The Brain Is Contained Within the Cranium, and Constitutes the Upper, Greatly Expanded Part of the Central Nervous System
The brain is contained within the cranium, and constitutes the upper, greatly expanded part of the central nervous system. Henry Gray (1918) Looking through the gray outer layer of the cortex, you can see a mass of white matter. At the center is a cluster of large nuclei, including the basal ganglia, the hippocampi, the amygdalae, and two egg-shaped structures at the very center, barely visible in this figure, the thalami. The thalami rest on the lower brainstem (dark and light blue). You can also see the pituitary gland in front (beige), and the cerebellum at the rear of the brain (pink). In this chapter we will take these structures apart and re-build them from the bottom up. 09_P375070_Ch05.indd 126 1/29/2010 4:08:25 AM CHAPTER 5 The brain OUTLINE 1.0 Introduction 127 3.2 Output and input: the front-back division 143 1.1 The nervous system 128 3.3 The major lobes: visible and hidden 145 1.2 The geography of the brain 129 3.4 The massive interconnectivity of the cortex and thalamus 149 2.0 Growing a brain from the bottom up 133 3.5 The satellites of the subcortex 151 2.1 Evolution and personal history are expressed in the brain 133 4.0 Summary 153 2.2 Building a brain from bottom to top 134 5.0 Chapter review 153 3.0 From ‘ where ’ to ‘ what ’ : the functional 5.1 Study questions 153 roles of brain regions 136 5.2 Drawing exercises 153 3.1 The cerebral hemispheres: the left-right division 136 1.0 INTRODUCTION found. -

Reading Speech from Still and Moving Faces: the Neural Substrates of Visible Speech
Reading Speech from Still and Moving Faces: The Neural Substrates of Visible Speech Gemma A. Calvert1 and Ruth Campbell2 Downloaded from http://mitprc.silverchair.com/jocn/article-pdf/15/1/57/1757731/089892903321107828.pdf by guest on 18 May 2021 Abstract & Speech is perceived both by ear and by eye. Unlike heard regions including the left inferior frontal (Broca’s) area, left speech, some seen speech gestures can be captured in superior temporal sulcus (STS), and left supramarginal gyrus stilled image sequences. Previous studies have shown that in (the dorsal aspect of Wernicke’s area). Stilled speech hearing people, natural time-varying silent seen speech can sequences also generated activation in the ventral premotor access the auditory cortex (left superior temporal regions). cortex and anterior inferior parietal sulcus bilaterally. Using functional magnetic resonance imaging (fMRI), the Moving faces generated significantly greater cortical activa- present study explored the extent to which this circuitry was tion than stilled face sequences, and in similar regions. activated when seen speech was deprived of its time-varying However, a number of differences between stilled and moving characteristics. speech were also observed. In the visual cortex, stilled faces In the scanner, hearing participants were instructed to generated relatively more activation in primary visual regions look for a prespecified visible speech target sequence (‘‘voo’’ (V1/V2), while visual movement areas (V5/MT+) were or ‘‘ahv’’) among other monosyllables. In one condition, the activated to a greater extent by moving faces. Cortical regions image sequence comprised a series of stilled key frames activated more by naturally moving speaking faces included showing apical gestures (e.g., separate frames for ‘‘v’’ and the auditory cortex (Brodmann’s Areas 41/42; lateral parts of ‘‘oo’’ [from the target] or ‘‘ee’’ and ‘‘m’’ [i.e., from Heschl’s gyrus) and the left STS and inferior frontal gyrus. -

Distinct Hippocampal Functional Networks Revealed by Tractography-Based Parcellation
Brain Struct Funct DOI 10.1007/s00429-015-1084-x ORIGINAL ARTICLE Distinct hippocampal functional networks revealed by tractography-based parcellation 1 2,5 3 2,4 Areeba Adnan • Alexander Barnett • Massieh Moayedi • Cornelia McCormick • 2,5 2,5 Melanie Cohn • Mary Pat McAndrews Received: 27 February 2015 / Accepted: 7 July 2015 Ó Springer-Verlag Berlin Heidelberg 2015 Abstract Recent research suggests the anterior and pos- parahippocampal, inferior temporal and fusiform gyri and terior hippocampus form part of two distinct functional the precuneus, predicted interindividual relational memory neural networks. Here we investigate the structural under- performance. These findings provide important support for pinnings of this functional connectivity difference using the integration of structural and functional connectivity in diffusion-weighted imaging-based parcellation. Using this understanding the brain networks underlying episodic technique, we substantiated that the hippocampus can be memory. parcellated into distinct anterior and posterior segments. These structurally defined segments did indeed show dif- Keywords DTI Á fMRI Á Hippocampus Á Recognition ferent patterns of resting state functional connectivity, in that memory Á Networks the anterior segment showed greater connectivity with temporal and orbitofrontal cortex, whereas the posterior segment was more highly connected to medial and lateral Introduction parietal cortex. Furthermore, we showed that the posterior hippocampal connectivity to memory processing regions, There is a growing consensus for the specialization of hip- including the dorsolateral prefrontal cortex, pocampal function along the anterior–posterior axis, arising out of differential involvement in large-scale brain networks (Poppenk et al. 2013). Functional neuroimaging has shown A. Adnan, A. Barnett and M. Moayedi contributed equally to this work and share first authorship. -

Are the Neural Correlates of Consciousness in the Front Or in the Back of the Cerebral Cortex?
bioRxiv preprint doi: https://doi.org/10.1101/118273; this version posted March 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence Melanie Boly1,2*, Marcello Massimini3,4, Naotsugu Tsuchiya5, Bradley R. Postle2,6, Christof Koch7, Giulio Tononi2* 1 Department of Neurology, University of Wisconsin, Madison, WI, 53705 USA 2 Department of Psychiatry, University of Wisconsin, Madison, WI, 53719 USA 3 Department of Biomedical and Clinical Sciences ‘Luigi Sacco’, University of Milan, Milan, 20157, Italy 4 Instituto Di Ricovero e Cura a Carattere Scientifico, Fondazione Don Carlo Gnocchi, Milan, 20148, Italy 6 Department of Psychology, Monash University, Melbourne, 3168, Australia 6 Department of Psychology, University of Wisconsin, Madison, WI, 53705 USA 7 Allen Institute for Brain Science, Seattle, WA, 98109 USA Abbreviated title: Is consciousness in the front versus the back of corteX? Correspondence to: Melanie Boly: [email protected] or Giulio Tononi: [email protected]. Address: 6001 Research Park Boulevard, Madison WI 53719 Number of pages: 25 Number of figures: 5 Number of words for Abstract: 45; number of words for Main teXt: 3750 Conflicts of Interest: The authors declare no conflicts of interest. Acknowledgments: This work was supported by NIH/NINDS 1R03NS096379 (to M.B.), by the Tiny Blue Dot Foundation and the Distinguished Chair in Consciousness Science (University of Wisconsin) (to G.T.), and by the James S. -
Interaction of Inferior Temporal Cortex with Frontal Cortex and Basal Forebrain: Double Dissociation in Strategy Implementation and Associative Learning
The Journal of Neuroscience, August 15, 2002, 22(16):7288–7296 Interaction of Inferior Temporal Cortex with Frontal Cortex and Basal Forebrain: Double Dissociation in Strategy Implementation and Associative Learning David Gaffan,1 Alexander Easton,2 and Amanda Parker2 1Department of Experimental Psychology, Oxford University, Oxford OX1 3UD, United Kingdom, and 2School of Psychology, Nottingham University, Nottingham NG7 2RD, United Kingdom Macaque monkeys learned a strategy task in which two groups TSϩAMϩFX cannot be generally attributed to the partial tem- of visual objects needed to be treated differently, one with poral–frontal disconnection that this lesion creates, and there- persistent and one with sporadic object choices, to obtain food fore support the hypothesis that the amnesic effects of this rewards. After preoperative training, they were divided into two lesion are caused primarily by the disconnection of temporal surgical groups of three monkeys each. One group received cortex from ascending inputs from the basal forebrain. The crossed unilateral removals of frontal cortex and inferior tem- results also show that temporal–frontal interaction in strategy poral cortex (IT ϫ FC) and were severely impaired in performing implementation does not require those routes of temporal– the strategy task. The other group received bilateral transection frontal interaction that are interrupted in TSϩAMϩFX, and of anterior temporal stem, amygdala, and fornix (TSϩAMϩFX) therefore support the hypothesis that projections to other pos- and were unimpaired in performing the strategy task. Subse- terior cortical areas allow temporal and frontal cortex to interact quently the same animals were tested in visual object–reward with each other by multisynaptic corticocortical routes in strat- association learning. -

Neurodynamics and Connectivity During Facial Fear Perception
www.nature.com/scientificreports OPEN Neurodynamics and connectivity during facial fear perception: The role of threat exposure and signal Received: 23 June 2017 Accepted: 9 January 2018 congruity Published: xx xx xxxx Cody A. Cushing1, Hee Yeon Im1,2, Reginald B. Adams Jr.3, Noreen Ward1, Daniel N. Albohn3, Troy G. Steiner3 & Kestutis Kveraga 1,2 Fearful faces convey threat cues whose meaning is contextualized by eye gaze: While averted gaze is congruent with facial fear (both signal avoidance), direct gaze (an approach signal) is incongruent with it. We have previously shown using fMRI that the amygdala is engaged more strongly by fear with averted gaze during brief exposures. However, the amygdala also responds more to fear with direct gaze during longer exposures. Here we examined previously unexplored brain oscillatory responses to characterize the neurodynamics and connectivity during brief (~250 ms) and longer (~883 ms) exposures of fearful faces with direct or averted eye gaze. We performed two experiments: one replicating the exposure time by gaze direction interaction in fMRI (N = 23), and another where we confrmed greater early phase locking to averted-gaze fear (congruent threat signal) with MEG (N = 60) in a network of face processing regions, regardless of exposure duration. Phase locking to direct-gaze fear (incongruent threat signal) then increased signifcantly for brief exposures at ~350 ms, and at ~700 ms for longer exposures. Our results characterize the stages of congruent and incongruent facial threat signal processing and show that stimulus exposure strongly afects the onset and duration of these stages. When we look at a face, we can glean a wealth of information, such as age, sex, health, afective state, and atten- tional focus. -

Experience Sampling During Fmri Reveals Default Network and Executive System Contributions to Mind Wandering
Experience sampling during fMRI reveals default network and executive system contributions to mind wandering Kalina Christoffa,1, Alan M. Gordonb, Jonathan Smallwoodc, Rachelle Smitha, and Jonathan W. Schoolerc aDepartment of Psychology, University of British Columbia, Vancouver, BC, Canada V6Z 2R8; bDepartment of Psychology, Stanford University, CA 94305; and cDepartment of Psychology, University of California, Santa Barbara, CA 93106 Edited by Michael I. Posner, University of Oregon, Eugene, OR, and approved March 27, 2009 (received for review February 9, 2009) Although mind wandering occupies a large proportion of our there were no online measures of mind wandering taken during waking life, its neural basis and relation to ongoing behavior scanning, it is possible that the observed default network recruit- remain controversial. We report an fMRI study that used experi- ment could be due to factors other than mind wandering. In this ence sampling to provide an online measure of mind wandering vein, Gilbert et al. (9) recently argued that instead of mind during a concurrent task. Analyses focused on the interval of time wandering, activations in the medial PFC part of the default immediately preceding experience sampling probes demonstrate network may reflect stimulus-related thought such as enhanced activation of default network regions during mind wandering, a watchfulness toward the external environment that is also likely to finding consistent with theoretical accounts of default network occur during highly practiced tasks. functions. Activation in medial prefrontal default network regions A key objective of the present study was to provide a direct was observed both in association with subjective self-reports of empirical test for the hypothesis that default network recruitment, mind wandering and an independent behavioral measure (perfor- including medial PFC regions, occurs during the precise moments mance errors on the concurrent task). -

Rebecca D. Burwell, Ph.D. Curriculum Vitae
Rebecca D. Burwell, Ph.D. Curriculum Vitae Rebecca D. Burwell, Ph.D. Professor of Psychology and Neuroscience Department of Cognitive, Linguistic, and Psychological Sciences Brown University [email protected] Professional Appointments: Professor of Psychology (secondary appointment in Neuroscience), Brown University, 2006- present Professor of Neuroscience (secondary appointment), Brown University, 2006-present Associate Professor of Neuroscience (secondary appointment), Brown University, 2003-2006 Associate Professor of Psychology, Brown University, 2002-2006 Assistant Professor of Psychology, Brown University, 1996-2002 Postdoctoral Fellow and Lecturer, Center for Behavioral Neuroscience, The State University of New York at Stony Brook, 1993-1996 Postdoctoral Research Associate, Laboratory for Neuronal Structure and Function, The Salk Institute for Biological Studies, 1992-1993 Education Ph.D., The University of North Carolina at Chapel Hill, 1992 Experimental and Biological Psychology Program Thesis title: The Effects of Aging on Brain Dopamine Systems and Behavior M.A., The University of North Carolina at Chapel Hill, 1989 Clinical Psychology Program Thesis title: The Relationship Between Age-related Deficits in Spatial Learning and Diurnal Rhythms B.A., Southern Methodist University, 1974 Academic Honors National Merit Scholarship, 1971-1974 University Scholarship, SMU, 1973-1974 James R. Kenan Fellowship, UNC, 08/87-05/88 NSF Predoctoral Fellowship, 06/88-05/91 APA Division 20 Student Research Award, 1991 NIH Predoctoral Fellowship, 06/91-08/92 NIMH Postdoctoral Fellowship, 12/92-11/94 McDonnell-Pew Fellowship to Cold Spring Harbor Biology of Memory Course, Summer 1993 NIMH Postdoctoral Fellowship, 03/95-02/96 Salomon Award: The Postrhinal Cortex and Context Conditioning, 1997-1999 NSF Career Development Award: Cognitive Functions of the Postrhinal Cortex, 1999-2003 Karen T. -

Area 7 Pdf, Epub, Ebook
AREA 7 PDF, EPUB, EBOOK Matthew Reilly | 512 pages | 17 Feb 2003 | St Martin's Press | 9780312983222 | English | New York, United States Area 7 PDF Book Schofield gets back to Area 7, but the prisoners that were there for scientific research get out, and make the remaining Air Force and Marines fight to the death. Return to Play Activities Waiver. Apr 30, Alex rated it it was amazing Shelves: in Original Title. Please reference the Developmental Disabilities Services Coverage and Limitations Handbook for more details on services offered under this program. Friday 9 October Forever glad the bears survived. Thursday 20 August Bellingham Bay Fishery see 1 below for boundaries Aug. This is the second book I've read by Matthew Reilly and just like Ice Station the first book in the Scarecrow series it's an action packed read from start to finish! Most of these people die. Melbourne , Victoria , Australia. Monday 29 June Parahippocampal gyrus anterior Entorhinal cortex Perirhinal cortex Postrhinal cortex Posterior parahippocampal gyrus Prepyriform area. Friday 29 May And in the process, he wrote a story that definitely gave me something to worry about. Shane Schofield 4 books. Why, because they have access to many of the installations containing some of this countries greatest tactical assets: high tech planes and ballistic missiles. Area 7 Shane Schofield 2 by Matthew Reilly. For smelt: Jig gear may be used 7 days a week. Sunday 5 July Visual cortex 17 Cuneus Lingual gyrus Calcarine sulcus. Trump trailing behind Biden in funding, weeks before Election Day, new filings show. This book SHOULD be read only if you are waiting in the airport to catch a long-delayed flight, or while pursuing some other similar pursuit. -

Posterior Cortex Epilepsy: Diagnostic Considerations and Surgical Outcome
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Seizure 18 (2009) 288–292 Contents lists available at ScienceDirect Seizure journal homepage: www.elsevier.com/locate/yseiz Posterior cortex epilepsy: Diagnostic considerations and surgical outcome Tao Yu, Yuping Wang, Guojun Zhang, Lixin Cai, Wei Du, Yongjie Li * Beijing Institute of Functional Neurosurgery, Xuanwu Hospital, Capital Medical University, China ARTICLE INFO SUMMARY Article history: Purpose: To assess the role of various pre-surgical evaluations in posterior cortex epilepsy (PCE) and its Received 15 January 2008 surgical outcome. Received in revised form 12 November 2008 Methods: A retrospective analysis of clinical and laboratory data from 43 PCE patients referred for Accepted 20 November 2008 surgery was performed. The diagnosis was established by standard pre-surgical evaluation modalities including semiology, MRI, interictal and ictal scalp video-EEG as well as additional intracranial EEG Keywords: monitoring in selected cases. Posterior cortex Results: The 43 patients included 11 parietal lobe epilepsies, 13 occipital lobe epilepsies, and 19 patients Epilepsy surgery with seizures originating from parieto-occipito-posterior temporal cortex. Thirty-three (76.7%) patients Surgical outcome experienced at least one type of aura. Seventeen patients showed complex focal seizures, which were followed by secondarily generalized tonic–clonic seizures in seven of them; eighteen patients predominantly showed simple motor seizures (clonic seizures in 6, tonic seizures in 7, and versive seizures in 5). Long-term visual field deficits were observed in 8 patients. Other transient neurological deficits occurred in 7 patients. All patients received the follow-up study lasting 1–5 years, and achieved Engel’s I in 26 cases, II in 5, III in 5, and IV–V in 7. -

Cortical Connectivity Maps Reveal Anatomically Distinct Areas in the Parietal Cortex of the Rat
UC Irvine UC Irvine Previously Published Works Title Cortical connectivity maps reveal anatomically distinct areas in the parietal cortex of the rat. Permalink https://escholarship.org/uc/item/01d2v835 Journal Frontiers in neural circuits, 8(JAN) ISSN 1662-5110 Authors Wilber, Aaron A Clark, Benjamin J Demecha, Alexis J et al. Publication Date 2014 DOI 10.3389/fncir.2014.00146 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California ORIGINAL RESEARCH ARTICLE published: 05 January 2015 NEURAL CIRCUITS doi: 10.3389/fncir.2014.00146 Cortical connectivity maps reveal anatomically distinct areas in the parietal cortex of the rat Aaron A. Wilber 1,2*†, Benjamin J. Clark 1,3*†, Alexis J. Demecha 1, Lilia Mesina 1, Jessica M. Vos 1 and Bruce L. McNaughton 1,2 1 Canadian Centre for Behavioural Neuroscience, The University of Lethbridge, Lethbridge, AB, Canada 2 Department of Neurobiology and Behavior, University of California, Irvine, CA, USA 3 Department of Psychology, The University of New Mexico, Albuquerque, NM, USA Edited by: A central feature of theories of spatial navigation involves the representation of spatial Charles F.Stevens, The Salk relationships between objects in complex environments. The parietal cortex has long Institute for Biological Studies, USA been linked to the processing of spatial visual information and recent evidence from Reviewed by: single unit recording in rodents suggests a role for this region in encoding egocentric Richard Hahnloser, ETH University Zurich, Switzerland and world-centered frames. The rat parietal cortex can be subdivided into four distinct Ya-tang Li, California Institute of rostral-caudal and medial-lateral regions, which includes a zone previously characterized as Technology, USA secondary visual cortex. -

REVIEW ARTICLE the Retrosplenial-Parietal Network and Reference Frame Coordination for Spatial Navigation
REVIEW ARTICLE The Retrosplenial-Parietal Network and Reference Frame Coordination for Spatial Navigation Benjamin J. Clark1, Christine M. Simmons2, Laura E. Berkowitz1, Aaron A. Wilber2 1Department of Psychology, University of New Mexico, Albuquerque, New Mexico 2Department of Psychology, Program in Neuroscience, Florida State University, Tallahassee, Florida Main text: 35 Pages, 2 figures Key Words: spatial navigation, reference frame, parietal cortex, posterior parietal cortex, retrosplenial cortex To whom correspondence should be addressed: [email protected] [email protected] Acknowledgments: The manuscript was written while the authors were supported by grants from NIAAA P50AA022534 and R21AA024983 to BJC and NIA R00AG049090 to AAW. 2 Abstract The retrosplenial cortex is anatomically positioned to integrate sensory, motor, and visual information and is thought to have an important role in processing spatial information and guiding behavior through complex environments. Anatomical and theoretical work has argued that the retrosplenial cortex participates in spatial behavior in concert with its primary input, the parietal cortex. Although the nature of this interactions is unknown, the central position is that the functional connectivity is hierarchical with egocentric spatial information processed at parietal cortex, and higher-level allocentric mappings generated in the retrosplenial cortex. Here, we review the evidence supporting this proposal. We begin by summarizing the key anatomical features of the retrosplenial-parietal network, and then review studies investigating the neural correlates of these regions during spatial behavior. Our summary of this literature suggests that the retrosplenial-parietal circuitry does not represent a strict hierarchical parcellation of function between the two regions, but instead a heterogeneous mixture of egocentric-allocentric coding and integration across frames of reference.