The Energy Meaning of Boltzmann's Constant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dimensional Analysis and the Theory of Natural Units
LIBRARY TECHNICAL REPORT SECTION SCHOOL NAVAL POSTGRADUATE MONTEREY, CALIFORNIA 93940 NPS-57Gn71101A NAVAL POSTGRADUATE SCHOOL Monterey, California DIMENSIONAL ANALYSIS AND THE THEORY OF NATURAL UNITS "by T. H. Gawain, D.Sc. October 1971 lllp FEDDOCS public This document has been approved for D 208.14/2:NPS-57GN71101A unlimited release and sale; i^ distribution is NAVAL POSTGRADUATE SCHOOL Monterey, California Rear Admiral A. S. Goodfellow, Jr., USN M. U. Clauser Superintendent Provost ABSTRACT: This monograph has been prepared as a text on dimensional analysis for students of Aeronautics at this School. It develops the subject from a viewpoint which is inadequately treated in most standard texts hut which the author's experience has shown to be valuable to students and professionals alike. The analysis treats two types of consistent units, namely, fixed units and natural units. Fixed units include those encountered in the various familiar English and metric systems. Natural units are not fixed in magnitude once and for all but depend on certain physical reference parameters which change with the problem under consideration. Detailed rules are given for the orderly choice of such dimensional reference parameters and for their use in various applications. It is shown that when transformed into natural units, all physical quantities are reduced to dimensionless form. The dimension- less parameters of the well known Pi Theorem are shown to be in this category. An important corollary is proved, namely that any valid physical equation remains valid if all dimensional quantities in the equation be replaced by their dimensionless counterparts in any consistent system of natural units. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Useful Constants
Appendix A Useful Constants A.1 Physical Constants Table A.1 Physical constants in SI units Symbol Constant Value c Speed of light 2.997925 × 108 m/s −19 e Elementary charge 1.602191 × 10 C −12 2 2 3 ε0 Permittivity 8.854 × 10 C s / kgm −7 2 μ0 Permeability 4π × 10 kgm/C −27 mH Atomic mass unit 1.660531 × 10 kg −31 me Electron mass 9.109558 × 10 kg −27 mp Proton mass 1.672614 × 10 kg −27 mn Neutron mass 1.674920 × 10 kg h Planck constant 6.626196 × 10−34 Js h¯ Planck constant 1.054591 × 10−34 Js R Gas constant 8.314510 × 103 J/(kgK) −23 k Boltzmann constant 1.380622 × 10 J/K −8 2 4 σ Stefan–Boltzmann constant 5.66961 × 10 W/ m K G Gravitational constant 6.6732 × 10−11 m3/ kgs2 M. Benacquista, An Introduction to the Evolution of Single and Binary Stars, 223 Undergraduate Lecture Notes in Physics, DOI 10.1007/978-1-4419-9991-7, © Springer Science+Business Media New York 2013 224 A Useful Constants Table A.2 Useful combinations and alternate units Symbol Constant Value 2 mHc Atomic mass unit 931.50MeV 2 mec Electron rest mass energy 511.00keV 2 mpc Proton rest mass energy 938.28MeV 2 mnc Neutron rest mass energy 939.57MeV h Planck constant 4.136 × 10−15 eVs h¯ Planck constant 6.582 × 10−16 eVs k Boltzmann constant 8.617 × 10−5 eV/K hc 1,240eVnm hc¯ 197.3eVnm 2 e /(4πε0) 1.440eVnm A.2 Astronomical Constants Table A.3 Astronomical units Symbol Constant Value AU Astronomical unit 1.4959787066 × 1011 m ly Light year 9.460730472 × 1015 m pc Parsec 2.0624806 × 105 AU 3.2615638ly 3.0856776 × 1016 m d Sidereal day 23h 56m 04.0905309s 8.61640905309 -

Introduction to Dimensional Analysis2 Usually, a Certain Physical Quantity
(2) Introduction to Dimensional Analysis2 Usually, a certain physical quantity, say, length or mass, is expressed by a number indicating how many times it is as large as a certain unit quantity. Therefore, the statement that the length of this stick is 3 does not make sense; we must say, with a certain unit, for example, that the length of this stick is 3 m. A number with a unit is a meaningless number as the number itself (3m and 9.8425¢ ¢ ¢ feet are the same). That is, we may freely scale it through choosing an appropriate unit. In contrast, the statement that the ratio of the lengths of this and that stick is 4 makes sense independent of the choice of the unit. A quantity whose numerical value does not depend on the choice of units is calle a dimensionless quantity. The number 4 here is dimensionless, and has an absolute meaning in contrast to the previous number 3. The statement that the length of this stick is 3m depends not only on the property of the stick but also on how we observe (or describe) it. In contrast, the statement that the ratio of the lengths is 4 is independent of the way we describe it. A formula describing a relation among several physical quantities is actually a relation among several numbers. If the physical relation holds `apart from us,' or in other words, is independent of the way we describe it, then whether the relation holds or not should not depend on the choice of the units or such `convenience to us.' A relation correct only when the length is measured in meters is hardly a good objective relation among physical quantities (it misses an important universal property of the law of physics, or at least very inconvenient). -

Summary of Dimensionless Numbers of Fluid Mechanics and Heat Transfer 1. Nusselt Number Average Nusselt Number: Nul = Convective
Jingwei Zhu http://jingweizhu.weebly.com/course-note.html Summary of Dimensionless Numbers of Fluid Mechanics and Heat Transfer 1. Nusselt number Average Nusselt number: convective heat transfer ℎ퐿 Nu = = L conductive heat transfer 푘 where L is the characteristic length, k is the thermal conductivity of the fluid, h is the convective heat transfer coefficient of the fluid. Selection of the characteristic length should be in the direction of growth (or thickness) of the boundary layer; some examples of characteristic length are: the outer diameter of a cylinder in (external) cross flow (perpendicular to the cylinder axis), the length of a vertical plate undergoing natural convection, or the diameter of a sphere. For complex shapes, the length may be defined as the volume of the fluid body divided by the surface area. The thermal conductivity of the fluid is typically (but not always) evaluated at the film temperature, which for engineering purposes may be calculated as the mean-average of the bulk fluid temperature T∞ and wall surface temperature Tw. Local Nusselt number: hxx Nu = x k The length x is defined to be the distance from the surface boundary to the local point of interest. 2. Prandtl number The Prandtl number Pr is a dimensionless number, named after the German physicist Ludwig Prandtl, defined as the ratio of momentum diffusivity (kinematic viscosity) to thermal diffusivity. That is, the Prandtl number is given as: viscous diffusion rate ν Cpμ Pr = = = thermal diffusion rate α k where: ν: kinematic viscosity, ν = μ/ρ, (SI units : m²/s) k α: thermal diffusivity, α = , (SI units : m²/s) ρCp μ: dynamic viscosity, (SI units : Pa ∗ s = N ∗ s/m²) W k: thermal conductivity, (SI units : ) m∗K J C : specific heat, (SI units : ) p kg∗K ρ: density, (SI units : kg/m³). -

International System of Units (SI)
International System of Units (SI) National Institute of Standards and Technology (www.nist.gov) Multiplication Prefix Symbol factor 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 * hecto h 101 * deka da 10-1 * deci d 10-2 * centi c 10-3 milli m 10-6 micro µ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a * Prefixes of 100, 10, 0.1, and 0.01 are not formal SI units From: Downs, R.J. 1988. HortScience 23:881-812. International System of Units (SI) Seven basic SI units Physical quantity Unit Symbol Length meter m Mass kilogram kg Time second s Electrical current ampere A Thermodynamic temperature kelvin K Amount of substance mole mol Luminous intensity candela cd Supplementary SI units Physical quantity Unit Symbol Plane angle radian rad Solid angle steradian sr From: Downs, R.J. 1988. HortScience 23:881-812. International System of Units (SI) Derived SI units with special names Physical quantity Unit Symbol Derivation Absorbed dose gray Gy J kg-1 Capacitance farad F A s V-1 Conductance siemens S A V-1 Disintegration rate becquerel Bq l s-1 Electrical charge coulomb C A s Electrical potential volt V W A-1 Energy joule J N m Force newton N kg m s-2 Illumination lux lx lm m-2 Inductance henry H V s A-1 Luminous flux lumen lm cd sr Magnetic flux weber Wb V s Magnetic flux density tesla T Wb m-2 Pressure pascal Pa N m-2 Power watt W J s-1 Resistance ohm Ω V A-1 Volume liter L dm3 From: Downs, R.J. -

Supplementary Notes on Natural Units Units
Physics 8.06 Feb. 1, 2005 SUPPLEMENTARY NOTES ON NATURAL UNITS These notes were prepared by Prof. Jaffe for the 1997 version of Quantum Physics III. They provide background and examples for the material which will be covered in the first lecture of the 2005 version of 8.06. The primary motivation for the first lecture of 8.06 is that physicists think in natural units, and it’s about time that you start doing so too. Next week, we shall begin studying the physics of charged particles mov- ing in a uniform magnetic field. We shall be lead to consider Landau levels, the integer quantum hall effect, and the Aharonov-Bohm effect. These are all intrinsically and profoundly quantum mechanical properties of elemen- tary systems, and can each be seen as the starting points for vast areas of modern theoretical and experimental physics. A second motivation for to- day’s lecture on units is that if you were a physicist working before any of these phenomena had been discovered, you could perhaps have predicted im- portant aspects of each of them just by thinking sufficiently cleverly about natural units. Before getting to that, we’ll learn about the Chandrasekhar mass, namely the limiting mass of a white dwarf or neutron star, supported against collapse by what one might loosely call “Pauli pressure”. There too, natural units play a role, although it is too much to say that you could have predicted the Chandrasekhar mass only from dimensional analysis. What is correct is the statement that if you understood the physics well enough to realize 2 that Mchandrasekhar ∝ 1/mproton, then thinking about natural units would have been enough to get you to Chandrasekhar’s result. -

Thermodynamic Temperature
Thermodynamic temperature Thermodynamic temperature is the absolute measure 1 Overview of temperature and is one of the principal parameters of thermodynamics. Temperature is a measure of the random submicroscopic Thermodynamic temperature is defined by the third law motions and vibrations of the particle constituents of of thermodynamics in which the theoretically lowest tem- matter. These motions comprise the internal energy of perature is the null or zero point. At this point, absolute a substance. More specifically, the thermodynamic tem- zero, the particle constituents of matter have minimal perature of any bulk quantity of matter is the measure motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., non- mechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational motions” are almost always in the classical dynamic temperature is often also called absolute tem- regime. Translational motions are ordinary, whole-body perature, for two reasons: one, proposed by Kelvin, that movements in three-dimensional space in which particles it does not depend on the properties of a particular mate- move about and exchange energy in collisions. Figure 1 rial; two that it refers to an absolute zero according to the below shows translational motion in gases; Figure 4 be- properties of the ideal gas. low shows translational motion in solids. Thermodynamic temperature’s null point, absolute zero, is the temperature The International System of Units specifies a particular at which the particle constituents of matter are as close as scale for thermodynamic temperature. -

Chemical Engineering Thermodynamics
CHEMICAL ENGINEERING THERMODYNAMICS Andrew S. Rosen SYMBOL DICTIONARY | 1 TABLE OF CONTENTS Symbol Dictionary ........................................................................................................................ 3 1. Measured Thermodynamic Properties and Other Basic Concepts .................................. 5 1.1 Preliminary Concepts – The Language of Thermodynamics ........................................................ 5 1.2 Measured Thermodynamic Properties .......................................................................................... 5 1.2.1 Volume .................................................................................................................................................... 5 1.2.2 Temperature ............................................................................................................................................. 5 1.2.3 Pressure .................................................................................................................................................... 6 1.3 Equilibrium ................................................................................................................................... 7 1.3.1 Fundamental Definitions .......................................................................................................................... 7 1.3.2 Independent and Dependent Thermodynamic Properties ........................................................................ 7 1.3.3 Phases ..................................................................................................................................................... -
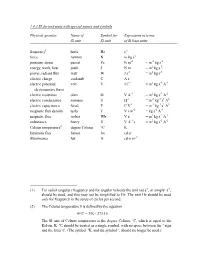
1.4.3 SI Derived Units with Special Names and Symbols
1.4.3 SI derived units with special names and symbols Physical quantity Name of Symbol for Expression in terms SI unit SI unit of SI base units frequency1 hertz Hz s-1 force newton N m kg s-2 pressure, stress pascal Pa N m-2 = m-1 kg s-2 energy, work, heat joule J N m = m2 kg s-2 power, radiant flux watt W J s-1 = m2 kg s-3 electric charge coulomb C A s electric potential, volt V J C-1 = m2 kg s-3 A-1 electromotive force electric resistance ohm Ω V A-1 = m2 kg s-3 A-2 electric conductance siemens S Ω-1 = m-2 kg-1 s3 A2 electric capacitance farad F C V-1 = m-2 kg-1 s4 A2 magnetic flux density tesla T V s m-2 = kg s-2 A-1 magnetic flux weber Wb V s = m2 kg s-2 A-1 inductance henry H V A-1 s = m2 kg s-2 A-2 Celsius temperature2 degree Celsius °C K luminous flux lumen lm cd sr illuminance lux lx cd sr m-2 (1) For radial (angular) frequency and for angular velocity the unit rad s-1, or simply s-1, should be used, and this may not be simplified to Hz. The unit Hz should be used only for frequency in the sense of cycles per second. (2) The Celsius temperature θ is defined by the equation θ/°C = T/K - 273.15 The SI unit of Celsius temperature is the degree Celsius, °C, which is equal to the Kelvin, K. -

The International System of Units (SI)
The International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 330 2008 Edition Barry N. Taylor and Ambler Thompson, Editors NIST SPECIAL PUBLICATION 330 2008 EDITION THE INTERNATIONAL SYSTEM OF UNITS (SI) Editors: Barry N. Taylor Physics Laboratory Ambler Thompson Technology Services National Institute of Standards and Technology Gaithersburg, MD 20899 United States version of the English text of the eighth edition (2006) of the International Bureau of Weights and Measures publication Le Système International d’ Unités (SI) (Supersedes NIST Special Publication 330, 2001 Edition) Issued March 2008 U.S. DEPARTMENT OF COMMERCE, Carlos M. Gutierrez, Secretary NATIONAL INSTITUTE OF STANDARDS AND TECHNOLOGY, James Turner, Acting Director National Institute of Standards and Technology Special Publication 330, 2008 Edition Natl. Inst. Stand. Technol. Spec. Pub. 330, 2008 Ed., 96 pages (March 2008) CODEN: NSPUE2 WASHINGTON 2008 Foreword The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant system used in science, the SI is rapidly becoming the dominant measurement system used in international commerce. In recognition of this fact and the increasing global nature of the marketplace, the Omnibus Trade and Competitiveness Act of 1988, which changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. industry increase its competitiveness, designates “the metric system of measurement as the preferred system of weights and measures for United States trade and commerce.” The definitive international reference on the SI is a booklet published by the International Bureau of Weights and Measures (BIPM, Bureau International des Poids et Mesures) and often referred to as the BIPM SI Brochure. -

EL Light Output Definition.Pdf
GWENT GROUP ADVANCED MATERIAL SYSTEMS Luminance The candela per square metre (cd/m²) is the SI unit of luminance; nit is a non-SI name also used for this unit. It is often used to quote the brightness of computer displays, which typically have luminance’s of 50 to 300 nits (the sRGB spec for monitor’s targets 80 nits). Modern flat-panel (LCD and plasma) displays often exceed 300 cd/m² or 300 nits. The term is believed to come from the Latin "nitere" = to shine. Candela per square metre 1 Kilocandela per square metre 10-3 Candela per square centimetre 10-4 Candela per square foot 0,09 Foot-lambert 0,29 Lambert 3,14×10-4 Nit 1 Stilb 10-4 Luminance is a photometric measure of the density of luminous intensity in a given direction. It describes the amount of light that passes through or is emitted from a particular area, and falls within a given solid angle. The SI unit for luminance is candela per square metre (cd/m2). The CGS unit of luminance is the stilb, which is equal to one candela per square centimetre or 10 kcd/m2 Illuminance The lux (symbol: lx) is the SI unit of illuminance and luminous emittance. It is used in photometry as a measure of the intensity of light, with wavelengths weighted according to the luminosity function, a standardized model of human brightness perception. In English, "lux" is used in both singular and plural. Microlux 1000000 Millilux 1000 Lux 1 Kilolux 10-3 Lumen per square metre 1 Lumen per square centimetre 10-4 Foot-candle 0,09 Phot 10-4 Nox 1000 In photometry, illuminance is the total luminous flux incident on a surface, per unit area.