The Normal Pancreas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Cholecystokinin Expression in the Developing and Regenerating Pancreas and Intestine
233 Cholecystokinin expression in the developing and regenerating pancreas and intestine G Liu, S V Pakala, D Gu, T Krahl, L Mocnik and N Sarvetnick Department of Immunology, Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA (Requests for offprints should be addressed to N Sarvetnick; Email: [email protected]) Abstract In developmental terms, the endocrine system of neither NOD mice continued this pattern. By contrast, in IFN- the gut nor the pancreatic islets has been characterized transgenic mice, CCK expression was suppressed from fully. Little is known about the involvement of cholecysto- birth to 3 months of age in the pancreata but not intestines. kinin (CCK), a gut hormone, involved in regulating the However, by 5 months of age, CCK expression appeared secretion of pancreatic hormones, and pancreatic growth. in the regenerating pancreatic ductal region of IFN- Here, we tracked CCK-expressing cells in the intestines transgenic mice. In the intestine, CCK expression per- and pancreata of normal mice (BALB/c), Non Obese sisted from fetus to adulthood and was not influenced Diabetic (NOD) mice and interferon (IFN)- transgenic by IFN-. Intestinal cells expressing CCK did not mice, which exhibit pancreatic regeneration, during em- co-express glucagon, suggesting that these cells are bryonic development, the postnatal period and adulthood. phenotypically distinct from CCK-expressing cells in We also questioned whether IFN- influences the expres- the pancreatic islets, and the effect of IFN- on sion of CCK. The results from embryonic day 16 showed CCK varies depending upon the cytokine’s specific that all three strains had CCK in the acinar region of microenvironment. -

Papilla with Separate Bile and Pancreatic Duct Orifices
JOP. J Pancreas (Online) 2013 May 10; 14(3):302-303. MULTIMEDIA ARTICLE – Clinical Imaging Papilla with Separate Bile and Pancreatic Duct Orifices Surinder Singh Rana, Deepak Kumar Bhasin Department of Gastroenterology, Post Graduate Institute of Medical Education and Research (PGIMER). Chandigarh, India A 32-year-old male, a known case of alcohol related Conflict of interest The authors have no potential chronic non calcific pancreatitis, was referred to us for conflicts of interest pancreatic endotherapy for relief of intractable abdominal pain. The cross sectional imaging studies References had revealed an irregularly dilated main pancreatic duct. The examination of the major duodenal papilla 1. Silvis SE, Vennes JA, Dreyer M. Variation in the normal duodenal papilla. Gastrointest Endosc 1983; 29:132-133 [PMID; revealed the presence of two separate orifices at 6852473] endoscopic retrograde cholangiopancreatography (ERCP) (Image). The cranial orifice was located at 11- 12 clock position whereas the caudal orifice was located at 4-5 clock position. The caudal orifice was selectively cannulated and the injection of the contrast revealed presence of an irregularly dilated main pancreatic duct. The cannula and the guide wire introduced through the caudal orifice selectively entered the pancreatic duct and did not come out through the cranial orifice. During ERCP, bile could be seen coming out of the cranial orifice, confirming it to be the orifice of common bile duct. Following selective cannulation of the main pancreatic duct, a 5-Fr stent was placed into the pancreatic duct. Following this, the patient had complete pain relief and is planned for further sessions of pancreatic endotherapy along with pancreatic sphincterotomy. -
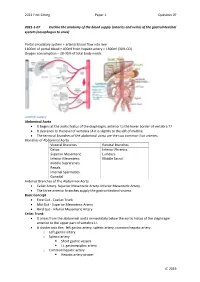
Arteries and Veins) of the Gastrointestinal System (Oesophagus to Anus)
2021 First Sitting Paper 1 Question 07 2021-1-07 Outline the anatomy of the blood supply (arteries and veins) of the gastrointestinal system (oesophagus to anus) Portal circulatory system + arterial blood flow into liver 1100ml of portal blood + 400ml from hepatic artery = 1500ml (30% CO) Oxygen consumption – 20-35% of total body needs Arterial Supply Abdominal Aorta • It begins at the aortic hiatus of the diaphragm, anterior to the lower border of vertebra T7. • It descends to the level of vertebra L4 it is slightly to the left of midline. • The terminal branches of the abdominal aorta are the two common iliac arteries. Branches of Abdominal Aorta Visceral Branches Parietal Branches Celiac. Inferior Phrenics. Superior Mesenteric. Lumbars Inferior Mesenteric. Middle Sacral. Middle Suprarenals. Renals. Internal Spermatics. Gonadal Anterior Branches of The Abdominal Aorta • Celiac Artery. Superior Mesenteric Artery. Inferior Mesenteric Artery. • The three anterior branches supply the gastrointestinal viscera. Basic Concept • Fore Gut - Coeliac Trunk • Mid Gut - Superior Mesenteric Artery • Hind Gut - Inferior Mesenteric Artery Celiac Trunk • It arises from the abdominal aorta immediately below the aortic hiatus of the diaphragm anterior to the upper part of vertebra LI. • It divides into the: left gastric artery, splenic artery, common hepatic artery. o Left gastric artery o Splenic artery ▪ Short gastric vessels ▪ Lt. gastroepiploic artery o Common hepatic artery ▪ Hepatic artery proper JC 2019 2021 First Sitting Paper 1 Question 07 • Left hepatic artery • Right hepatic artery ▪ Gastroduodenal artery • Rt. Gastroepiploic (gastro-omental) artery • Sup pancreatoduodenal artery • Supraduodenal artery Oesophagus • Cervical oesophagus - branches from inferior thyroid artery • Thoracic oesophagus - branches from bronchial arteries and aorta • Abd. -

Redalyc.Accessory Hepatic Artery: Incidence and Distribution
Jornal Vascular Brasileiro ISSN: 1677-5449 [email protected] Sociedade Brasileira de Angiologia e de Cirurgia Vascular Brasil Dutta, Sukhendu; Mukerjee, Bimalendu Accessory hepatic artery: incidence and distribution Jornal Vascular Brasileiro, vol. 9, núm. 1, 2010, pp. 25-27 Sociedade Brasileira de Angiologia e de Cirurgia Vascular São Paulo, Brasil Available in: http://www.redalyc.org/articulo.oa?id=245016483014 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative ORIGINAL ARTICLE Accessory hepatic artery: incidence and distribution Artéria hepática acessória: incidência e distribuição Sukhendu Dutta,1 Bimalendu Mukerjee2 Abstract Resumo Background: Anatomic variations of the hepatic arteries are com- Contexto: As variações anatômicas das artérias hepáticas são co- mon. Preoperative identification of these variations is important to pre- muns. A identificação pré-operatória dessas variações é importante para vent inadvertent injury and potentially lethal complications during open prevenir lesão inadvertida e complicações potencialmente letais durante and endovascular procedures. procedimentos abertos e endovasculares. Objective: To evaluate the incidence, extra-hepatic course, and Objetivo: Avaliar a incidência, o trajeto extra-hepático e a presen- presence of side branches of accessory hepatic arteries, defined as an ad- ça de ramos laterais das artérias hepáticas acessórias definidas como um ditional arterial supply to the liver in the presence of normal hepatic ar- suprimento arterial adicional para o fígado na presença de artéria hepática tery. normal. Métodos: Oitenta e quatro cadáveres humanos masculinos foram Methods: Eighty-four human male cadavers were dissected using dissecados através de laparotomia mediana transperitoneal. -

Vessels and Circulation
CARDIOVASCULAR SYSTEM OUTLINE 23.1 Anatomy of Blood Vessels 684 23.1a Blood Vessel Tunics 684 23.1b Arteries 685 23.1c Capillaries 688 23 23.1d Veins 689 23.2 Blood Pressure 691 23.3 Systemic Circulation 692 Vessels and 23.3a General Arterial Flow Out of the Heart 693 23.3b General Venous Return to the Heart 693 23.3c Blood Flow Through the Head and Neck 693 23.3d Blood Flow Through the Thoracic and Abdominal Walls 697 23.3e Blood Flow Through the Thoracic Organs 700 Circulation 23.3f Blood Flow Through the Gastrointestinal Tract 701 23.3g Blood Flow Through the Posterior Abdominal Organs, Pelvis, and Perineum 705 23.3h Blood Flow Through the Upper Limb 705 23.3i Blood Flow Through the Lower Limb 709 23.4 Pulmonary Circulation 712 23.5 Review of Heart, Systemic, and Pulmonary Circulation 714 23.6 Aging and the Cardiovascular System 715 23.7 Blood Vessel Development 716 23.7a Artery Development 716 23.7b Vein Development 717 23.7c Comparison of Fetal and Postnatal Circulation 718 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch23_683-723.indd 683 2/14/11 4:31 PM 684 Chapter Twenty-Three Vessels and Circulation lood vessels are analogous to highways—they are an efficient larger as they merge and come closer to the heart. The site where B mode of transport for oxygen, carbon dioxide, nutrients, hor- two or more arteries (or two or more veins) converge to supply the mones, and waste products to and from body tissues. The heart is same body region is called an anastomosis (ă-nas ′tō -mō′ sis; pl., the mechanical pump that propels the blood through the vessels. -

Arterial Arcades of Pancreas and Their Variations Chavan NN*, Wabale RN**
International J. of Healthcare and Biomedical Research, Volume: 03, Issue: 02, January 2015, Pages 23-33 Original article: Arterial arcades of Pancreas and their variations Chavan NN*, Wabale RN** [*Assistant Professor, ** Professor and Head] Department of Anatomy, Rural Medical College, PIMS, Loni , Tal. Rahata, Dist. Ahmednagar, Maharashtra, Pin - 413736. Corresponding author: Dr Chavan NM Abstract: Introduction : Pancreas is a highly vascular organ supplied by number of arteries and arterial arcades which provide blood supply to the organ. Arteries contributing to the arterial arcades are celiac and superior mesenteric arteries forming anterior and posterior arcades. These vascular arcades lie upon the surface of the pancreas but also supply the duodenal wall and are the chief obstacles to complete pancreatectomy without duodenectomy. Knowledge of variations of upper abdominal arteries is important while dealing with gastric and duodenal ulcers, biliary tract surgeries and mobilization of the head of the pancreas, as bleeding is one of the complications of these surgeries. During pancreaticoduodenectomies or lymph node resection procedures, these arcades are liable to injuries. Material and methods : Study was conducted on 50 specimens of pancreas removed enbloc from cadavers to study variations in the arcade. Observation and result : Anterior arterial arcade was present in 98% specimens and absent in 2%. It was formed by anterior superior pancreaticoduodenal artery(ASPDA) and anterior inferior pancreaticoduodenal artery(AIPDA) in 92%, Anterior superior pancreaticoduodenal artery (ASPDA), Anterior inferior pancreaticoduodenal artery (AIPDA) and Right dorsal pancreatic artery (Rt.DPA) in 2%, Anterior superior pancreaticoduodenal artery (ASPDA) only in 2%, Anterior superior pancreaticoduodenal artery (ASPDA) and Posterior inferior pancreaticoduodenal artery (PIPDA) in 2%, Arcade was absent and Anterior superior pancreaticoduodenal artery (ASPDA) gave branches in 2%. -

Portal Hypertensionand Its Radiological Investigation
Postgrad Med J: first published as 10.1136/pgmj.39.451.299 on 1 May 1963. Downloaded from POSTGRAD. MED. J. (I963), 39, 299 PORTAL HYPERTENSION AND ITS RADIOLOGICAL INVESTIGATION J. H. MIDDLEMISS, M.D., F.F.R., D.M.R.D. F. G. M. Ross, M.B., B.Ch., B.A.O., F.F.R., D.M.R.D. From the Department of Radiodiagnosis, United Bristol Hospitals PORTAL hypertension is a condition in which there branch of the portal vein but may drain into the right is an blood in the branch. abnormally high pressure Small veins which are present on the serosal surface portal system of veins which eventually leads to of the liver and in the surrounding peritoneal folds splenomegaly and in chronic cases, to haematem- draining the diaphragm and stomach are known as esis and melaena. accessory portal veins. They may unite with the portal The circulation is in that it vein or enter the liver independently. portal unique The hepatic artery arises normally from the coeliac exists between two sets of capillaries, i.e. the axis but it may arise as a separate trunk from the aorta. capillaries of the spleen, pancreas, gall-bladder It runs upwards and to the right and divides into a and most of the gastro-intestinal tract on the left and right branch before entering the liver at the one hand and the sinusoids of the liver on the porta hepatis. The venous return starts as small thin-walled branches other hand. The liver parallels the lungs in that in the centre of the lobules in the liver. -

Epithelium 2 : Glandular Epithelium Histology Laboratory -‐ Year 1, Fall Term Dr
Epithelium 2 : Glandular Epithelium Histology Laboratory -‐ Year 1, Fall Term Dr. Heather Yule ([email protected]) October 21, 2014 Slides for study: 75 (Salivary Gland), 355 (Pancreas Tail), 48 (Atrophic Mammary Gland), 49 (Active Mammary Gland) and 50 (Resting Mammary Gland) Electron micrographs for : study EM: Serous acinus in parotid gland EM: Mucous acinus in mixed salivary gland EM: Pancreatic acinar cell Main Objective: Understand key histological features of glandular epithelium and relate structure to function. Specific Objectives: 1. Describe key histological differences between endocrine and exocrine glands including their development. 2. Compare three modes of secretion in glands; holocrine, apocrine and merocrine. 3. Explain the functional significance of polarization of glandular epithelial cells. 4. Define the terms parenchyma, stroma, mucous acinus, serous acinus and serous a demilune and be able to them identify in glandular tissue. 5. Distinguish exocrine and endocrine pancreas. 6. Compare the histology of resting, lactating and postmenopausal mammary glands. Keywords: endocrine gland, exocrine gland, holocrine, apocrine, merocrine, polarity, parenchyma, stroma, acinus, myoepithelial cell, mucous gland, serous gland, mixed or seromucous gland, serous demilune, exocrine pancreas, endocrine pancreas (pancreatic islets), resting mammary gland, lactating mammary gland, postmenopausal mammary gland “This copy is made solely for your personal use for research, private study, education, parody, satire, criticism, or review -

An Analysis of Benign Human Prostate Offers Insights Into the Mechanism
www.nature.com/scientificreports OPEN An analysis of benign human prostate ofers insights into the mechanism of apocrine secretion Received: 12 March 2018 Accepted: 22 February 2019 and the origin of prostasomes Published: xx xx xxxx Nigel J. Fullwood 1, Alan J. Lawlor2, Pierre L. Martin-Hirsch3, Shyam S. Matanhelia3 & Francis L. Martin 4 The structure and function of normal human prostate is still not fully understood. Herein, we concentrate on the diferent cell types present in normal prostate, describing some previously unreported types and provide evidence that prostasomes are primarily produced by apocrine secretion. Patients (n = 10) undergoing TURP were prospectively consented based on their having a low risk of harbouring CaP. Scanning electron microscopy and transmission electron microscopy was used to characterise cell types and modes of secretion. Zinc levels were determined using Inductively Coupled Plasma Mass Spectrometry. Although merocrine secretory cells were noted, the majority of secretory cells appear to be apocrine; for the frst time, we clearly show high-resolution images of the stages of aposome secretion in human prostate. We also report a previously undescribed type of epithelial cell and the frst ultrastructural image of wrapping cells in human prostate stroma. The zinc levels in the tissues examined were uniformly high and X-ray microanalysis detected zinc in merocrine cells but not in prostasomes. We conclude that a signifcant proportion of prostasomes, possibly the majority, are generated via apocrine secretion. This fnding provides an explanation as to why so many large proteins, without a signal peptide sequence, are present in the prostatic fuid. Tere are many complications associated with the prostate from middle age onwards, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa). -

Endocrine Pancreatic Tumors: Ultrastructure
ANNALS OF CLINICAL AND LABORATORY SCIENCE, Vol. 10, No. 1 Copyright© 1980, Institute for Clinical Science, Inc. Endocrine Pancreatic Tumors: Ultrastructure MERY KOSTIANOVSKY, M.D. Department of Pathology, Thomas Jefferson University, Philadelphia, PA 19107 ABSTRACT Endocrine pancreatic tumors are frequently multicellular and produce several hormones and peptides. A review of the basic concepts of hormone secretion, pancreatic islet cell composition and ultrastructural make-up of tumors is presented. The importance of correlating ultrastructural, immuno- cytochemical and biochemical studies of these tumors is emphasized. Introduction Morphofunctional Aspects of Pancreatic Islets During the last few years a great amount of information was accumulated regarding At the present time four different types the mechanisms of synthesis, storage and of cells have been described in the pan release of hormones.14,28,31 The use of ex creatic islets,17,33 each having a specific perimental in vitro m odels21,25,26,28 was secretory product (table I). A variety of very helpful in clarifying the participation other cells possibly exists, although of different organelles in the biosynthesis, further identification is awaited. By light cellular “packaging” and emyocytosis of microscopy, it is not possible to distin the secretory products. In a review, Lacy31 guish one type of cell from the other. has proposed a working model for hor Histochemical procedures are of help, mone secretion, describing the sim however, and B cells are easily stained ilarities between different endocrine with aldehyde fuchsin. The dicferent pro glands. Some of this information was ob cedures and empiric nature oi the silver tained through the studies of endocrine stain22 added confusion in the nomen pancreatic tumors as in the case of the clature of the cells (as seen in table I) discovery of pro-insulin in a beta cell where the same cell has been described adenoma.62 The purpose of this paper is to by different names. -

Epithelium and Glands
EPITHELIUM AND GLANDS OBJECTIVES: After completing this exercise, students should be able to do the following: 1. Identify glands. 2. Classify glands based on secretory type. ASSIGNMENT FOR TODAY'S LABORATORY GLASS SLIDES SL 111 (Trachea) cilia and unicellular glands (goblet cells) SL 019 (Jejunum, PAS) unicellular glands SL 092 (Submandibular gland) serous, mucous and demilune secretory units SL 093 (Sublingual gland) mucous secretory units POSTED ELECTRON MICROGRAPHS # 7 Organelles # 11 Desmosomes # 12 Epithelium # 13 Freeze-fracture Lab 5 Posted EMs; Lab 5 Posted EMs with some yellow labels SUPPLEMENTAL MATERIAL: SUPPLEMENTARY ELECTRON MICROGRAPHS Rhodin, J. A.G., An Atlas of Histology. Glands pp. 46 - 52 Copies of this text are on reserve in the HSL. Glandular epithelium is specialized for the production and secretion of products. The cells that form glands are usually cuboidal or columnar in shape. In this exercise we are emphasizing morphological differences in glands with respect to secretory products. A. UNICELLULAR GLANDS: SL 111 (low, high), (Trachea, H&E); SL 019 (oil), (Jejunum, PAS), for review. Goblet cells may be few or numerous and are found in epithelia of the respiratory and alimentary systems. The secretory product is emptied into the lumen of the organ rather than into ducts (J. Fig. 4-18, 15-24; R. 5.38, Plate 60) B. MULTICELLULAR GLANDS: In general these glands are formed by invagination, proliferation, and differentiation of the epithelium from which they are derived. Glands that maintain a connection with the surface epithelium through ducts are termed exocrine glands, whereas glands that have lost this connection, and secrete instead to blood vessels, are called endocrine glands (see J.