Pdcd10-Stk24/25 Complex Controls Kidney Water Reabsorption by Regulating Aqp2 Membrane Targeting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Detection of Aneuploidies by Paralogous Sequence Quantification S Deutsch, U Choudhury, G Merla, C Howald, a Sylvan, S E Antonarakis
908 J Med Genet: first published as 10.1136/jmg.2004.023184 on 9 December 2004. Downloaded from ORIGINAL ARTICLE Detection of aneuploidies by paralogous sequence quantification S Deutsch, U Choudhury, G Merla, C Howald, A Sylvan, S E Antonarakis ............................................................................................................................... J Med Genet 2004;41:908–915. doi: 10.1136/jmg.2004.023184 Background: Chromosomal aneuploidies are a common cause of congenital disorders associated with cognitive impairment and multiple dysmorphic features. Pre-natal diagnosis of aneuploidies is most See end of article for commonly performed by the karyotyping of fetal cells obtained by amniocentesis or chorionic villus authors’ affiliations sampling, but this method is labour intensive and requires about 14 days to complete. ....................... Methods: We have developed a PCR based method for the detection of targeted chromosome number Correspondence to: abnormalities termed paralogous sequence quantification (PSQ), based on the use of paralogous genes. Professor Stylianos E Paralogous sequences have a high degree of sequence identity, but accumulate nucleotide substitutions in Antonarakis, Department a locus specific manner. These sequence differences, which we term paralogous sequence mismatches of Genetic Medicine and Development, University of (PSMs), can be quantified using pyrosequencing technology, to estimate the relative dosage between Geneva Medical School, different chromosomes. We designed 10 assays for the detection of trisomies of chromosomes 13, 18, and GE 1211, Geneva, 21 and sex chromosome aneuploidies. Switzerland; Stylianos. antonarakis@medecine. Results: We evaluated the performance of this method on 175 DNAs, highly enriched for abnormal unige.ch samples. A correct and unambiguous diagnosis was given for 119 out of 120 aneuploid samples as well as for all the controls. -

Novel Mutation and Three Other Sequence Variants Segregating with Phenotype at Keratoconus 13Q32 Susceptibility Locus
European Journal of Human Genetics (2012) 20, 389–397 & 2012 Macmillan Publishers Limited All rights reserved 1018-4813/12 www.nature.com/ejhg ARTICLE Novel mutation and three other sequence variants segregating with phenotype at keratoconus 13q32 susceptibility locus Marta Czugala1,6, Justyna A Karolak1,6, Dorota M Nowak1, Piotr Polakowski2, Jose Pitarque3, Andrea Molinari3, Malgorzata Rydzanicz1, Bassem A Bejjani4, Beatrice YJT Yue5, Jacek P Szaflik2 and Marzena Gajecka*,1 Keratoconus (KTCN), a non-inflammatory corneal disorder characterized by stromal thinning, represents a major cause of corneal transplantations. Genetic and environmental factors have a role in the etiology of this complex disease. Previously reported linkage analysis revealed that chromosomal region 13q32 is likely to contain causative gene(s) for familial KTCN. Consequently, we have chosen eight positional candidate genes in this region: MBNL1, IPO5, FARP1, RNF113B, STK24, DOCK9, ZIC5 and ZIC2, and sequenced all of them in 51 individuals from Ecuadorian KTCN families and 105 matching controls. The mutation screening identified one mutation and three sequence variants showing 100% segregation under a dominant model with KTCN phenotype in one large Ecuadorian family. These substitutions were found in three different genes: c.2262A4C (p.Gln754His) and c.720+43A4GinDOCK9; c.2377-132A4CinIPO5 and c.1053+29G4CinSTK24. PolyPhen analyses predicted that c.2262A4C (Gln754His) is possibly damaging for the protein function and structure. Our results suggest that c.2262A4C (p.Gln754His) -
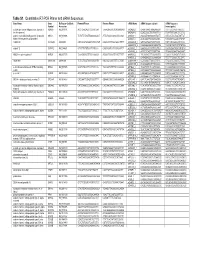
Table S1. Quantitative RT-PCR Primer and Sirna Sequences Gene Name
Table S1. Quantitative RT-PCR Primer and siRNA Sequences Gene Name Gene RefSeq or GenBank Forward Primer Reverse Primer siRNA Name siRNA Sequence (plus) siRNA Sequence Symbol Accession (minus/guide) a disintegrin and metalloproteinase domain 9 ADAM9 NM_003816 ACCTCAGCAGTTCCCATCAA TAAAGGAGGTGCAGGAGCAG siADAM9_1 GAGATTAACTAGAGAAAGA TCTTTCTCTAGTTAATCTC (meltrin gamma) siADAM9_2 GGAGGGAGTTCATAATTCA TGAATTATGAACTCCCTCC guanine nucleotide binding protein (G protein), GNAI3 NM_006496 TCTGTTATAGTTGGCGGCAGT ATTCTGCAAAGGCAAAAGGA siGNAI3_1 AGAACTAGCAGGAGTGATT AATCACTCCTGCTAGTTCT alpha inhibiting activity polypeptide 3 siGNAI3_2 ACACAGGTTCCAATACATA TATGTATTGGAACCTGTGT AA099748 AA099748 AA099748 CTACCACTGAGGTGTCCCTGT CAGCCATCTAACAGCATTTTT siAA099748_1 GTTAGATGGCTGATTAATA TATTAATCAGCCATCTAAC siAA099748_2 CGACAGAGGAGCAGCATTA TAATGCTGCTCCTCTGTCG calpain 12 CAPN12 NM_144691 GTCCTTCTGTCCCTCATCCA CAGCAGCTCCTCTGGAATCT siCAPN12_1 GGACACGCGTATTCCATCA TGATGGAATACGCGTGTCC siCAPN12_2 AATCCTCAGTTCCGTTTAA TTAAACGGAACTGAGGATT NMDA receptor regulated 1 NARG1 NM_057175 TAAAGGGAATTTGCCGAAGA AGCACTGGAGTTCGGTTTGT siNARG1_1 TGCGAGATCTTGAGGGTTA TAACCCTCAAGATCTCGCA siNARG1_2 GATAGGAGGTCCAAAAGAA TTCTTTTGGACCTCCTATC AK091308 AK091308 AK091308 TCTCCTGAATGGGAGGAATG GGCAACAGATGTTTCCTGTG siAK091308_1 CCAAAGACTTAGACTGTAA TTACAGTCTAAGTCTTTGG siAK091308_2 GCACAAAGCATTTACAACA TGTTGTAAATGCTTTGTGC serine/threonine kinase 24 (STE20 homolog, STK24 NM_003576 GCATCTGCCTTCCTTATCCA TGACAGTGTTTTGCCAGAGG siSTK24_1 TCGATTATCTCCATTCGGA TCCGAATGGAGATAATCGA yeast) siSTK24_2 CATCGGACTTGGACAGAAA -

Individualized Systems Medicine Strategy to Tailor Treatments for Patients with Chemorefractory Acute Myeloid Leukemia
Published OnlineFirst September 20, 2013; DOI: 10.1158/2159-8290.CD-13-0350 RESEARCH ARTICLE Individualized Systems Medicine Strategy to Tailor Treatments for Patients with Chemorefractory Acute Myeloid Leukemia Tea Pemovska 1 , Mika Kontro 2 , Bhagwan Yadav 1 , Henrik Edgren 1 , Samuli Eldfors1 , Agnieszka Szwajda 1 , Henrikki Almusa 1 , Maxim M. Bespalov 1 , Pekka Ellonen 1 , Erkki Elonen 2 , Bjørn T. Gjertsen5 , 6 , Riikka Karjalainen 1 , Evgeny Kulesskiy 1 , Sonja Lagström 1 , Anna Lehto 1 , Maija Lepistö1 , Tuija Lundán 3 , Muntasir Mamun Majumder 1 , Jesus M. Lopez Marti 1 , Pirkko Mattila 1 , Astrid Murumägi 1 , Satu Mustjoki 2 , Aino Palva 1 , Alun Parsons 1 , Tero Pirttinen 4 , Maria E. Rämet 4 , Minna Suvela 1 , Laura Turunen 1 , Imre Västrik 1 , Maija Wolf 1 , Jonathan Knowles 1 , Tero Aittokallio 1 , Caroline A. Heckman 1 , Kimmo Porkka 2 , Olli Kallioniemi 1 , and Krister Wennerberg 1 ABSTRACT We present an individualized systems medicine (ISM) approach to optimize cancer drug therapies one patient at a time. ISM is based on (i) molecular profi ling and ex vivo drug sensitivity and resistance testing (DSRT) of patients’ cancer cells to 187 oncology drugs, (ii) clinical implementation of therapies predicted to be effective, and (iii) studying consecutive samples from the treated patients to understand the basis of resistance. Here, application of ISM to 28 samples from patients with acute myeloid leukemia (AML) uncovered fi ve major taxonomic drug-response sub- types based on DSRT profi les, some with distinct genomic features (e.g., MLL gene fusions in subgroup IV and FLT3 -ITD mutations in subgroup V). Therapy based on DSRT resulted in several clinical responses. -

Mirna‑26A‑5P and Mir‑26B‑5P Inhibit the Proliferation of Bladder Cancer Cells by Regulating PDCD10
ONCOLOGY REPORTS 40: 3523-3532, 2018 miRNA‑26a‑5p and miR‑26b‑5p inhibit the proliferation of bladder cancer cells by regulating PDCD10 KE WU1*, XING-YU MU1*, JUN-TAO JIANG1, MING-YUE TAN1, REN-JIE WANG1, WEN-JIE ZHOU2, XIANG WANG1, YIN-YAN HE3, MING-QING LI2 and ZHI-HONG LIU1 1Department of Urology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200080; 2Laboratory for Reproductive Immunology, Hospital of Obstetrics and Gynecology, Fudan University, Shanghai 200011; 3Department of Obstetrics and Gynecology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200080, P.R. China Received October 11, 2017; Accepted September 4, 2018 DOI: 10.3892/or.2018.6734 Abstract. MicroRNA (miR)-26a-5p and miR-26b-5p consis- and miR-26b-5p were pivotal regulators in BC progression tently play an antitumor role in many types of cancers, but the by targeting the proliferation-related protein, PDCD10. The underlying mechanism remains unclear in bladder cancer (BC). miR-26-5p/PDCD10 interaction may provide important In the present study, we found that, in BC tissues, the levels of insight into the pathway of BC progression and present novel miR-26a-5p and miR-26b-5p were lower than in paired normal opportunities for future diagnosis and treatment strategies, tissues. The upregulation of miR‑26‑5p significantly inhibited especially for patients with high levels of PDCD10. the proliferation of BC cell lines (T24 and 5637). Bioinformatics analysis indicated that Programmed Cell Death 10 (PDCD10) Introduction was the downstream target gene of miR-26a-5p/miR-26b-5p, and this was ascertained by western blotting and quantitative Bladder cancer (BC) is one of the most important urinary real-time reverse transcription PCR (RT-qPCR). -

STK24 Antibody - C-Terminal Region Rabbit Polyclonal Antibody Catalog # AI16132
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 STK24 Antibody - C-terminal region Rabbit Polyclonal Antibody Catalog # AI16132 Specification STK24 Antibody - C-terminal region - Product Information Application WB Primary Accession Q9Y6E0 Other Accession NP_003567 Reactivity Human Host Rabbit Clonality Polyclonal Calculated MW 48kDa KDa STK24 Antibody - C-terminal region - Additional Information Host: Rabbit Target Name: STK24 Gene ID 8428 Sample Tissue: 293T Whole Cell lysates Antibody Dilution: 1.0μg/ml Alias Symbol STK24, MST3, STK3, Other Names STK24 Antibody - C-terminal region - Serine/threonine-protein kinase 24, Background 2.7.11.1, Mammalian STE20-like protein kinase 3, MST-3, STE20-like kinase MST3, Serine/threonine-protein kinase that acts on Serine/threonine-protein kinase 24 36 kDa both serine and threonine residues and subunit, Mammalian STE20-like protein promotes apoptosis in response to stress kinase 3 N-terminal, MST3/N, stimuli and caspase activation. Mediates Serine/threonine-protein kinase 24 12 kDa oxidative-stress- induced cell death by subunit, Mammalian STE20-like protein modulating phosphorylation of JNK1-JNK2 kinase 3 C-terminal, MST3/C, STK24, MST3, (MAPK8 and MAPK9), p38 (MAPK11, MAPK12, STK3 MAPK13 and MAPK14) during oxidative stress. Plays a role in a staurosporine-induced Format caspase- independent apoptotic pathway by Liquid. Purified antibody supplied in 1x PBS buffer with 0.09% (w/v) sodium azide and regulating the nuclear translocation of AIFM1 2% sucrose. and ENDOG and the DNase activity associated with ENDOG. Phosphorylates STK38L on Reconstitution & Storage 'Thr-442' and stimulates its kinase activity. Add 50 &mu, l of distilled water. -

MST3 (STK24) (NM 001032296) Human Tagged ORF Clone Lentiviral Particle Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC209117L4V MST3 (STK24) (NM_001032296) Human Tagged ORF Clone Lentiviral Particle Product data: Product Type: Lentiviral Particles Product Name: MST3 (STK24) (NM_001032296) Human Tagged ORF Clone Lentiviral Particle Symbol: STK24 Synonyms: HEL-S-95; MST3; MST3B; STE20; STK3 Vector: pLenti-C-mGFP-P2A-Puro (PS100093) ACCN: NM_001032296 ORF Size: 1293 bp ORF Nucleotide The ORF insert of this clone is exactly the same as(RC209117). Sequence: OTI Disclaimer: The molecular sequence of this clone aligns with the gene accession number as a point of reference only. However, individual transcript sequences of the same gene can differ through naturally occurring variations (e.g. polymorphisms), each with its own valid existence. This clone is substantially in agreement with the reference, but a complete review of all prevailing variants is recommended prior to use. More info OTI Annotation: This clone was engineered to express the complete ORF with an expression tag. Expression varies depending on the nature of the gene. RefSeq: NM_001032296.2 RefSeq Size: 4624 bp RefSeq ORF: 1296 bp Locus ID: 8428 UniProt ID: Q9Y6E0, Q5U0E6, Q6P0Y1 Protein Families: Druggable Genome, Protein Kinase MW: 47.7 kDa Gene Summary: This gene encodes a serine/threonine protein kinase that functions upstream of mitogen- activated protein kinase (MAPK) signaling. The encoded protein is cleaved into two chains by caspases; the N-terminal fragment (MST3/N) translocates to the nucleus and promotes programmed cells death. -

Cerebral Cavernous Malformations: from Genes to Proteins to Disease
See the corresponding editorial in this issue, pp 119–121. J Neurosurg 116:122–132, 2012 Cerebral cavernous malformations: from genes to proteins to disease Clinical article DANIEL D. CAVALCANTI, M.D., M. YASHAR S. KALANI, M.D., PH.D., NIKOLAY L. MARTIROSYAN, M.D., JUSTIN EALES, B.S., ROBERT F. SPETZLER, M.D., AND MARK C. PREUL, M.D. Division of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona Over the past half century molecular biology has led to great advances in our understanding of angio- and vas- culogenesis and in the treatment of malformations resulting from these processes gone awry. Given their sporadic and familial distribution, their developmental and pathological link to capillary telangiectasias, and their observed chromosomal abnormalities, cerebral cavernous malformations (CCMs) are regarded as akin to cancerous growths. Although the exact pathological mechanisms involved in the formation of CCMs are still not well understood, the identification of 3 genetic loci has begun to shed light on key developmental pathways involved in CCM pathogen- esis. Cavernous malformations can occur sporadically or in an autosomal dominant fashion. Familial forms of CCMs have been attributed to mutations at 3 different loci implicated in regulating important processes such as proliferation and differentiation of angiogenic precursors and members of the apoptotic machinery. These processes are important for the generation, maintenance, and pruning of every vessel in the body. In this review the authors highlight the lat- est discoveries pertaining to the molecular genetics of CCMs, highlighting potential new therapeutic targets for the treatment of these lesions. -

STRIPAK Complexes in Cell Signaling and Cancer
Oncogene (2016), 1–9 © 2016 Macmillan Publishers Limited All rights reserved 0950-9232/16 www.nature.com/onc REVIEW STRIPAK complexes in cell signaling and cancer Z Shi1,2, S Jiao1 and Z Zhou1,3 Striatin-interacting phosphatase and kinase (STRIPAK) complexes are striatin-centered multicomponent supramolecular structures containing both kinases and phosphatases. STRIPAK complexes are evolutionarily conserved and have critical roles in protein (de) phosphorylation. Recent studies indicate that STRIPAK complexes are emerging mediators and regulators of multiple vital signaling pathways including Hippo, MAPK (mitogen-activated protein kinase), nuclear receptor and cytoskeleton remodeling. Different types of STRIPAK complexes are extensively involved in a variety of fundamental biological processes ranging from cell growth, differentiation, proliferation and apoptosis to metabolism, immune regulation and tumorigenesis. Growing evidence correlates dysregulation of STRIPAK complexes with human diseases including cancer. In this review, we summarize the current understanding of the assembly and functions of STRIPAK complexes, with a special focus on cell signaling and cancer. Oncogene advance online publication, 15 February 2016; doi:10.1038/onc.2016.9 INTRODUCTION in the central nervous system and STRN4 is mostly abundant in Recent proteomic studies identified a group of novel multi- the brain and lung, whereas STRN3 is ubiquitously expressed in 5–9 component complexes named striatin (STRN)-interacting phos- almost all tissues. STRNs share a -

STRIPAK Directs PP2A Activity Toward MAP4K4 to Promote Oncogenic Transformation
bioRxiv preprint doi: https://doi.org/10.1101/823096; this version posted October 29, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. STRIPAK directs PP2A activity toward MAP4K4 to promote oncogenic transformation Jong Wook Kim1,2,7,8*, Christian Berrios2,4*, Miju Kim1,2*, Amy E. Schade2,4*, Guillaume Adelmant5, Huwate Yeerna7, Emily Damato1, Amanda Balboni Iniguez1,6, Selene K. Swanson9, Laurence Florens9, Michael P. Washburn9.10, Kim Stegmaier1,6, Nathaniel S. Gray5, Pablo Tamayo7,8, Ole Gjoerup2, Jarrod A. Marto5, James A. DeCaprio2,3,4,†, William C. Hahn1, 2, 3,† 1Broad Institute of Harvard and MIT, Cambridge MA. 2Department of Medical Oncology, Dana-Farber Cancer Institute, Boston MA. 3Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston MA. 4Program in Virology, Graduate School of Arts and Sciences, Harvard University, Cambridge, MA. 5Department of Cancer Biology and Blais Proteomics Center, Dana-Farber Cancer Institute, Boston MA. 6Department of Pediatric Oncology, Dana-Farber Cancer Institute, Boston MA. 7Division of Medical Genetics, School of Medicine, UC San Diego, CA. 8Moores Cancer Center, University of California, San Diego, CA. 9Stowers Institute for Medical Research, Kansas City, MO 64110 10Department of Pathology and Laboratory Medicine, University of Kansas Medical Center, Kansas City, KS 66160 * These authors contributed equally to the work. †Co-senior authors 1 bioRxiv preprint doi: https://doi.org/10.1101/823096; this version posted October 29, 2019. -

HHS Public Access Author Manuscript
HHS Public Access Author manuscript Author Manuscript Author ManuscriptGenet Med Author Manuscript. Author manuscript; Author Manuscript available in PMC 2016 March 01. Published in final edited form as: Genet Med. 2015 March ; 17(3): 188–196. doi:10.1038/gim.2014.97. EXCEPTIONAL AGGRESSIVENESS OF CEREBRAL CAVERNOUS MALFORMATION DISEASE ASSOCIATED WITH PDCD10 MUTATIONS Robert Shenkar, PhD#1, Changbin Shi, MD, PhD#1, Tania Rebeiz, MD2, Rebecca A. Stockton, PhD3, David A. McDonald, PhD4,5, Abdul Ghani Mikati, MD1, Lingjiao Zhang, MS1, Cecilia Austin, BS1, Amy L. Akers, PhD6, Carol J. Gallione, BA4, Autumn Rorrer, BA4, Murat Gunel, MD7, Wang Min, PhD8, Jorge Marcondes De Souza, MD, PhD9, Connie Lee, PsyD6, Douglas A. Marchuk, PhD#4, and Issam A. Awad, MD, MSc#1,2 1Neurovascular Surgery Program, Section of Neurosurgery, The University of Chicago Medicine, Chicago, IL 60637, USA 2Department of Neurology, The University of Chicago Medicine, Chicago, IL 60637, USA 3Department of Pediatrics, University of California at Los Angeles, Torrance, CA 90502, USA 4Department of Molecular Genetics and Microbiology, Duke University, Durham, NC 27710, USA 5Center for Science, Math and Technology Education, North Carolina Central University, Durham, NC 27707, USA 6Angioma Alliance, Norfolk, VA 23510, USA 7Departments of Neurosurgery and Neurobiology, Yale University, New Haven, CT 06520, USA 8Department of Pathology, Yale University, New Haven, CT 06520, USA 9Department of Neurosurgery, School of Medicine, Federal University of Rio De Janeiro, Rio de Janeiro, Brazil # These authors contributed equally to this work. Abstract Purpose—The phenotypic manifestations of cerebral cavernous malformation (CCM) disease caused by rare PDCD10 mutations have not been systematically examined, and a mechanistic link to Rho kinase (ROCK) mediated hyperpermeability, a potential therapeutic target, has not been established. -

Identification of Endogenous Adenomatous Polyposis Coli Interaction Partners and B-Catenin– Independent Targets by Proteomics
Published OnlineFirst June 3, 2019; DOI: 10.1158/1541-7786.MCR-18-1154 Cancer "-omics" Molecular Cancer Research Identification of Endogenous Adenomatous Polyposis Coli Interaction Partners and b-Catenin– Independent Targets by Proteomics Olesja Popow1,2,3,Joao~ A. Paulo2, Michael H. Tatham4, Melanie S. Volk3, Alejandro Rojas-Fernandez5, Nicolas Loyer3, Ian P. Newton3, Jens Januschke3, Kevin M. Haigis1,6, and Inke Nathke€ 3 Abstract Adenomatous Polyposis Coli (APC) is the most frequently tion between endogenous MINK1 and APC and further mutated gene in colorectal cancer. APC negatively regulates confirmed the negative, and b-catenin–independent, regu- the Wnt signaling pathway by promoting the degradation of lation of MINK1 by APC. Increased Mink1/Msn levels were b-catenin, but the extent to which APC exerts Wnt/b-cate- also observed in mouse intestinal tissue and Drosophila nin–independent tumor-suppressive activity is unclear. To follicular cells expressing mutant Apc/APC when compared identify interaction partners and b-catenin–independent with wild-type tissue/cells. Collectively, our results highlight targets of endogenous, full-length APC, we applied label- the extent and importance of Wnt-independent APC func- free and multiplexed tandem mass tag-based mass spec- tions in epithelial biology and disease. trometry. Affinity enrichment-mass spectrometry identified more than 150 previously unidentified APC interaction Implications: The tumor-suppressive function of APC, the partners. Moreover, our global proteomic analysis revealed most frequently mutated gene in colorectal cancer, is mainly that roughly half of the protein expression changes that attributed to its role in b-catenin/Wnt signaling. Our study occur in response to APC loss are independent of b-catenin.