Bioflavonoid Profile of Exotic Citrus Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thesis of Potentially Sweet Dihydrochalcone Glycosides
University of Bath PHD The synthesis of potentially sweet dihydrochalcone glycosides. Noble, Christopher Michael Award date: 1974 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 05. Oct. 2021 THE SYNTHESIS OF POTBTTIALLY SWEET DIHYDROCHALCOITB GLYCOSIDES submitted by CHRISTOPHER MICHAEL NOBLE for the degree of Doctor of Philosophy of the University of Bath. 1974 COPYRIGHT Attention is drawn to the fact that copyright of this thesis rests with its author.This copy of the the sis has been supplied on condition that anyone who con sults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be pub lished without the prior written consent of the author. -

Treatment Protocol Copyright © 2018 Kostoff Et Al
Prevention and reversal of Alzheimer's disease: treatment protocol Copyright © 2018 Kostoff et al PREVENTION AND REVERSAL OF ALZHEIMER'S DISEASE: TREATMENT PROTOCOL by Ronald N. Kostoffa, Alan L. Porterb, Henry. A. Buchtelc (a) Research Affiliate, School of Public Policy, Georgia Institute of Technology, USA (b) Professor Emeritus, School of Public Policy, Georgia Institute of Technology, USA (c) Associate Professor, Department of Psychiatry, University of Michigan, USA KEYWORDS Alzheimer's Disease; Dementia; Text Mining; Literature-Based Discovery; Information Technology; Treatments Prevention and reversal of Alzheimer's disease: treatment protocol Copyright © 2018 Kostoff et al CITATION TO MONOGRAPH Kostoff RN, Porter AL, Buchtel HA. Prevention and reversal of Alzheimer's disease: treatment protocol. Georgia Institute of Technology. 2018. PDF. https://smartech.gatech.edu/handle/1853/59311 COPYRIGHT AND CREATIVE COMMONS LICENSE COPYRIGHT Copyright © 2018 by Ronald N. Kostoff, Alan L. Porter, Henry A. Buchtel Printed in the United States of America; First Printing, 2018 CREATIVE COMMONS LICENSE This work can be copied and redistributed in any medium or format provided that credit is given to the original author. For more details on the CC BY license, see: http://creativecommons.org/licenses/by/4.0/ This work is licensed under a Creative Commons Attribution 4.0 International License<http://creativecommons.org/licenses/by/4.0/>. DISCLAIMERS The views in this monograph are solely those of the authors, and do not represent the views of the Georgia Institute of Technology or the University of Michigan. This monograph is not intended as a substitute for the medical advice of physicians. The reader should regularly consult a physician in matters relating to his/her health and particularly with respect to any symptoms that may require diagnosis or medical attention. -

Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid
Known Host Plants of Huanglongbing (HLB) and Asian Citrus Psyllid Diaphorina Liberibacter citri Plant Name asiaticus Citrus Huanglongbing Psyllid Aegle marmelos (L.) Corr. Serr.: bael, Bengal quince, golden apple, bela, milva X Aeglopsis chevalieri Swingle: Chevalier’s aeglopsis X X Afraegle gabonensis (Swingle) Engl.: Gabon powder-flask X Afraegle paniculata (Schum.) Engl.: Nigerian powder- flask X Atalantia missionis (Wall. ex Wight) Oliv.: see Pamburus missionis X X Atalantia monophylla (L.) Corr.: Indian atalantia X Balsamocitrus dawei Stapf: Uganda powder- flask X X Burkillanthus malaccensis (Ridl.) Swingle: Malay ghost-lime X Calodendrum capense Thunb.: Cape chestnut X × Citroncirus webberi J. Ingram & H. E. Moore: citrange X Citropsis gilletiana Swingle & M. Kellerman: Gillet’s cherry-orange X Citropsis schweinfurthii (Engl.) Swingle & Kellerm.: African cherry- orange X Citrus amblycarpa (Hassk.) Ochse: djerook leemo, djeruk-limau X Citrus aurantiifolia (Christm.) Swingle: lime, Key lime, Persian lime, lima, limón agrio, limón ceutí, lima mejicana, limero X X Citrus aurantium L.: sour orange, Seville orange, bigarde, marmalade orange, naranja agria, naranja amarga X Citrus depressa Hayata: shiikuwasha, shekwasha, sequasse X Citrus grandis (L.) Osbeck: see Citrus maxima X Citrus hassaku hort. ex Tanaka: hassaku orange X Citrus hystrix DC.: Mauritius papeda, Kaffir lime X X Citrus ichangensis Swingle: Ichang papeda X Citrus jambhiri Lushington: rough lemon, jambhiri-orange, limón rugoso, rugoso X X Citrus junos Sieb. ex Tanaka: xiang -

Plant Phenolics: Bioavailability As a Key Determinant of Their Potential Health-Promoting Applications
antioxidants Review Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications Patricia Cosme , Ana B. Rodríguez, Javier Espino * and María Garrido * Neuroimmunophysiology and Chrononutrition Research Group, Department of Physiology, Faculty of Science, University of Extremadura, 06006 Badajoz, Spain; [email protected] (P.C.); [email protected] (A.B.R.) * Correspondence: [email protected] (J.E.); [email protected] (M.G.); Tel.: +34-92-428-9796 (J.E. & M.G.) Received: 22 October 2020; Accepted: 7 December 2020; Published: 12 December 2020 Abstract: Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom that can be categorized as flavonoids and non-flavonoids. Interest in phenolic compounds has dramatically increased during the last decade due to their biological effects and promising therapeutic applications. In this review, we discuss the importance of phenolic compounds’ bioavailability to accomplish their physiological functions, and highlight main factors affecting such parameter throughout metabolism of phenolics, from absorption to excretion. Besides, we give an updated overview of the health benefits of phenolic compounds, which are mainly linked to both their direct (e.g., free-radical scavenging ability) and indirect (e.g., by stimulating activity of antioxidant enzymes) antioxidant properties. Such antioxidant actions reportedly help them to prevent chronic and oxidative stress-related disorders such as cancer, cardiovascular and neurodegenerative diseases, among others. Last, we comment on development of cutting-edge delivery systems intended to improve bioavailability and enhance stability of phenolic compounds in the human body. Keywords: antioxidant activity; bioavailability; flavonoids; health benefits; phenolic compounds 1. Introduction Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom with around 8000 different phenolic structures [1]. -

Bergamot) Juice Extracted from Three Different Cultivars Vincenzo Sicari*, Teresa Maria Pellicanò (Received December 14, 2015
Journal of Applied Botany and Food Quality 89, 171 - 175 (2016), DOI:10.5073/JABFQ.2016.089.021 Department of Agraria, University “Mediterranea” of Reggio Calabria, Reggio Calabria (RC), Italy Phytochemical properties and antioxidant potential from Citrus bergamia, Risso (bergamot) juice extracted from three different cultivars Vincenzo Sicari*, Teresa Maria Pellicanò (Received December 14, 2015) Summary Bergamot presents a unique profile of flavonoids and flavonoid Secondary substances occurring in plant-derived products possess glycosides in its juice, such as neoeriocitrin, neohesperidin, naringin, biological activity and thus may protect human beings from various narirutin (SICARI et al., 2015). Diets rich in flavonoids reduce post- diseases. ischemic miocardiac damage in rats (FACINO et al., 1999), coronaric The following physical and nutritional properties of three bergamot damage and the incidence of heart attacks in elderly man (HERTOG cultivars (Castagnaro, Fantastico and Femminello) were determined et al., 1993). and compared: pH, titratable acidity, vitamin C, total flavonoids, The major causes of cell damage following oxidative stress are total polyphenols, antocianyn, bioactive molecules and antioxidant reactive oxygen species (ROS). Reactive oxygen species (ROS) are capacity (ABTS and DPPH assay). The comparison data, were found produced as a normal product of plant cellular metabolism. Various to be statistically different. environmental stresses lead to excessive production of ROS causing In all juice samples analyzed the highest antioxidant capacity was progressive oxidative damage and ultimately cell death (SASTRE found in Castagnaro juice (64.21% I of DPPH and 1.97% I of ABTS) et al., 2000). compared to Fantastico (44.48% I of DPPH and 1.83% I of ABTS) After ingestion as food the flavanone glycosides are metabolized and Femminello (33.39% I of DPPH and 1.13% I of ABTS). -

Flavonoid Glucodiversification with Engineered Sucrose-Active Enzymes Yannick Malbert
Flavonoid glucodiversification with engineered sucrose-active enzymes Yannick Malbert To cite this version: Yannick Malbert. Flavonoid glucodiversification with engineered sucrose-active enzymes. Biotechnol- ogy. INSA de Toulouse, 2014. English. NNT : 2014ISAT0038. tel-01219406 HAL Id: tel-01219406 https://tel.archives-ouvertes.fr/tel-01219406 Submitted on 22 Oct 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Last name: MALBERT First name: Yannick Title: Flavonoid glucodiversification with engineered sucrose-active enzymes Speciality: Ecological, Veterinary, Agronomic Sciences and Bioengineering, Field: Enzymatic and microbial engineering. Year: 2014 Number of pages: 257 Flavonoid glycosides are natural plant secondary metabolites exhibiting many physicochemical and biological properties. Glycosylation usually improves flavonoid solubility but access to flavonoid glycosides is limited by their low production levels in plants. In this thesis work, the focus was placed on the development of new glucodiversification routes of natural flavonoids by taking advantage of protein engineering. Two biochemically and structurally characterized recombinant transglucosylases, the amylosucrase from Neisseria polysaccharea and the α-(1→2) branching sucrase, a truncated form of the dextransucrase from L. Mesenteroides NRRL B-1299, were selected to attempt glucosylation of different flavonoids, synthesize new α-glucoside derivatives with original patterns of glucosylation and hopefully improved their water-solubility. -

Glycosides in Lemon Fruit
Food Sci. Technol. Int. Tokyo, 4 (1), 48-53, 1998 Characteristics of Antioxidative Flavonoid Glycosides in Lemon Fruit Yoshiaki MIYAKE,1 Kanefumi YAMAMOT0,1 Yasujiro MORIMITSU2 and Toshihiko OSAWA2 * Central Research Laboratory of Pokka Corporation, Ltd., 45-2 Kumanosyo, Shikatsu-cho, Nishikasugai-gun, Aichi 481, Japan 2Department of Applied Biological Sciences, Nagoya University, Nagoya 46401, Japan Received June 12, 1997; Accepted September 27, 1997 We investigated the antioxidative flavonoid glycosides in the peel extract of lemon fruit (Citrus limon). Six flavanon glycosides: eriocitrin, neoeriocitrin, narirutin, naringin, hesperidin, and neohesperidin, and three flavone glycosides: diosmin, 6~-di- C-p-glucosyldiosmin (DGD), and 6- C-p-glucosyldiosmin (GD) were identified by high- performance liquid chromatography (HPLC) analysis. Their antioxidative activity was examined using a linoleic acid autoxidation system. The antioxidative activity of eriocitrin, neoeriocitrin and DGD was stronger than that of the others. Flavonoid glycosides were present primarily in the peel of lemon fruit. There was only a small difference in the content of the flavonoid glycosides of the lemon fruit juice from various sources and varieties. Lemon fruit contained abundant amounts of eriocitrin and hesperidin and also contained narirutin, diosmin, and DGD, but GD, neoeriocitrin, naringin, and neohesperidin were present only in trace amounts. The content of DGD, GD, and eriocitrin was especially abundant in lemons and limes; however, they were scarcely found in other citrus fruits. The content of flavonoid compounds in lemon juice obtained by an in-line extractor at a juice factory was more abundant than that obtained by hand-squeezing. These compounds were found to be stable even under heat treatment conditions (121'C, 15 min) in acidic solution. -
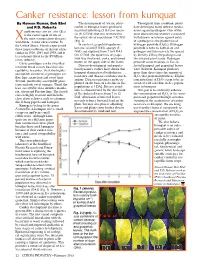
Canker Resistance: Lesson from Kumquat by Naveen Kumar, Bob Ebel the Development of Asiatic Citrus Throughout Their Evolution, Plants and P.D
Canker resistance: lesson from kumquat By Naveen Kumar, Bob Ebel The development of Asiatic citrus Throughout their evolution, plants and P.D. Roberts canker in kumquat leaves produced have developed many defense mecha- anthomonas citri pv. citri (Xcc) localized yellowing (5 DAI) or necro- nisms against pathogens. One of the is the causal agent of one of sis (9-12 DAI) that was restricted to most characteristic features associated the most serious citrus diseases the actual site of inoculation 7-12 DAI with disease resistance against entry X (Fig. 2). of a pathogen is the production of worldwide, Asiatic citrus canker. In the United States, Florida experienced In contrast, grapefruit epidermis hydrogen peroxide (H2O2). Hydrogen three major outbreaks of Asiatic citrus became raised (5 DAI), spongy (5 peroxide is toxic to both plant and canker in 1910, 1984 and 1995, and it DAI) and ruptured from 7 to 8 DAI. pathogen and thus restricts the spread is a constant threat to the $9 billion On 12 DAI, the epidermis of grape- by directly killing the pathogen and citrus industry. fruit was thickened, corky, and turned the infected plant tissue. Hydrogen Citrus genotypes can be classified brown on the upper side of the leaves. peroxide concentrations in Xcc-in- into four broad classes based on sus- Disease development and popula- fected kumquat and grapefruit leaves ceptibility to canker. First, the highly- tion dynamics studies have shown that were different. Kumquat produces susceptible commercial genotypes are kumquat demonstrated both disease more than three times the amount of Key lime, grapefruit and sweet lime. -

Part B Other Products Referred to in Article 2(1)
Part B Other products referred to in Article 2(1) Other products References to Part A to which the same MRLs apply (1) Main product of the group or subgroup Code number Category Code number or Common names/synonyms Scientific names Name of the group or subgroup 0110010-001 Natsudaidais Citrus natsudaidai 0110010-002 Shaddocks/pomelos Citrus maxima; syn: Citrus grandis 0110010-003 Sweeties/oroblancos Citrus grandis x Citrus paradisi 0110010 Grapefruits 0110010-004 Tangelolos Citrus paradisi x tangelo 0110010-005 Tangelos (except minneolas)/Ugli® Citrus tangelo 0110010-990 Other hybrids of Citrus paradisi , not elsewhere mentioned 0110020-001 Bergamots Citrus bergamia 0110020-002 Bitter oranges/sour oranges Citrus aurantium 0110020-003 Blood oranges Citrus sinensis 0110020 Oranges 0110020-004 Cara caras Citrus sinensis 0110020-005 Chinottos Citrus myrtifolia 0110020-006 Trifoliate oranges Poncirus trifoliata 0110020-990 Other hybrids of Citrus sinensis, not elsewhere mentioned 0110030-001 Buddha's hands/Buddha's fingers Citrus medica var. sarcodactyla 0110030 Lemons 0110030-002 Citrons Citrus medica 0110040-001 Indian sweet limes/Palestine sweet limes Citrus limettioides 0110040-002 Kaffir limes Citrus hystrix 0110040 Limes 0110040-003 Sweet limes/mosambis Citrus limetta 0110040-004 Tahiti limes Citrus latifolia 0110040-005 Limequats Citrus aurantiifolia x Fortunella spp. 0110050-001 Calamondins Citrus madurensis 0110050-002 Clementines Citrus clementina 0110050-003 Cleopatra mandarins Citrus reshni 0110050-004 Minneolas Citrus tangelo 0110050 Mandarins 0110050-005 Satsumas/clausellinas Citrus unshiu 0110050-006 Tangerines/dancy mandarins Citrus tangerina 0110050-007 Tangors Citrus nobilis 0110050-990 Other hybrids of Citrus reticulata , not elsewhere mentioned 0120010-001 Apricot kernels Armeniaca vulgaris; syn: Prunus armeniaca 0120010-002 Bitter almonds Amygdalus communis var. -

GRAS Notice (GRN) No. 719, Orange Pomace
GRAS Notice (GRN) No. 719 https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm SAFETY EVALUATION DOSSIER SUPPORTING A GENERALLY RECOGNIZED AS SAFE (GRAS) CONCLUSION FOR ORANGE POMACE SUBMITTED BY: PepsiCo, Inc. 700 Anderson Hill Road Purchase, NY 10577 SUBMITTED TO: U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food Additive Safety HFS-200 5100 Paint Branch Parkway College Park, MD 20740-3835 CONTACT FOR TECHNICAL OR OTHER INFORMATION: Andrey Nikiforov, Ph.D. Toxicology Regulatory Services, Inc. 154 Hansen Road, Suite 201 Charlottesville, VA 22911 July 3, 2017 Table of Contents Part 1. SIGNED STATEMENTS AND CERTIFICATION ...........................................................1 A. Name and Address of Notifier .............................................................................................1 B. Name of GRAS Substance ...................................................................................................1 C. Intended Use and Consumer Exposure ................................................................................1 D. Basis for GRAS Conclusion ................................................................................................2 E. Availability of Information ..................................................................................................3 Part 2. IDENTITY, METHOD OF MANUFACTURE, SPECIFICATIONS, AND PHYSICAL OR TECHNICAL EFFECT.................................................................................................4 -

PRUNING GUIDELINES Tools: Loppers, Saw, Clippers 10% Bleach and Water Solution
Varieties - choose one that you will want to eat often, as you will have them much of the year (Four Winds Citrus Variety Chart link in your Resources list) - certain citrus mature earlier than others (see early ripening handout) - Unique varieties: - Blood orange: red flesh is antioxidant rich. Often sweeter than other oranges - Yuzu: very little, but very flavorful juice used by chefs. Believed to be a cross between a sour mandarin and ichang papeda - Keiffir lime: regular lime with bumpy skin. Attractive tree with segmented leaves that are extremely fragrant and prized by chefs. - Buddhas hand: not much juice, but very fragrant pith and rind. Odd shaped and often used as an ornamental - Australian finger lime: oblong green lime with many small lime-flavored orbs inside. Called "citrus caviar" Standard varieties of citrus trees often grow to a height of 20 to 30 feet and the canopy -- or width of a tree -- can spread to 18 to 30 feet depending on the variety. Dwarf citrus trees are significantly shorter and narrower, which provides greater flexibility in planting location. Most varieties top out at 8 feet in height with a proportionally smaller canopy. Despite the differences in height and width, regular, semi-dwarf and dwarf citrus varieties produce the same size fruit. -First off DO NOT PRUNE! Those damaged leaves can actually provide protection for the plant until the air warms up. The plant needs to rally and recover. Pruning might just put it over the edge. Sometimes the plants must remain with that ‘raggedy’ appearance until as late as June and in some cases a full year. -

Citrus from Seed?
Which citrus fruits will come true to type Orogrande, Tomatera, Fina, Nour, Hernandina, Clementard.) from seed? Ellendale Tom McClendon writes in Hardy Citrus Encore for the South East: Fortune Fremont (50% monoembryonic) “Most common citrus such as oranges, Temple grapefruit, lemons and most mandarins Ugli Umatilla are polyembryonic and will come true to Wilking type. Because most citrus have this trait, Highly polyembryonic citrus types : will mostly hybridization can be very difficult to produce nucellar polyembryonic seeds that will grow true to type. achieve…. This unique characteristic Citrus × aurantiifolia Mexican lime (Key lime, West allows amateurs to grow citrus from seed, Indian lime) something you can’t do with, say, Citrus × insitorum (×Citroncirus webberii) Citranges, such as Rusk, Troyer etc. apples.” [12*] Citrus × jambhiri ‘Rough lemon’, ‘Rangpur’ lime, ‘Otaheite’ lime Monoembryonic (don’t come true) Citrus × limettioides Palestine lime (Indian sweet lime) Citrus × microcarpa ‘Calamondin’ Meyer Lemon Citrus × paradisi Grapefruit (Marsh, Star Ruby, Nagami Kumquat Redblush, Chironja, Smooth Flat Seville) Marumi Kumquat Citrus × sinensis Sweet oranges (Blonde, navel and Pummelos blood oranges) Temple Tangor Citrus amblycarpa 'Nasnaran' mandarin Clementine Mandarin Citrus depressa ‘Shekwasha’ mandarin Citrus karna ‘Karna’, ‘Khatta’ Poncirus Trifoliata Citrus kinokuni ‘Kishu mandarin’ Citrus lycopersicaeformis ‘Kokni’ or ‘Monkey mandarin’ Polyembryonic (come true) Citrus macrophylla ‘Alemow’ Most Oranges Citrus reshni ‘Cleopatra’ mandarin Changshou Kumquat Citrus sunki (Citrus reticulata var. austera) Sour mandarin Meiwa Kumquat (mostly polyembryonic) Citrus trifoliata (Poncirus trifoliata) Trifoliate orange Most Satsumas and Tangerines The following mandarin varieties are polyembryonic: Most Lemons Dancy Most Limes Emperor Grapefruits Empress Tangelos Fairchild Kinnow Highly monoembryonic citrus types: Mediterranean (Avana, Tardivo di Ciaculli) Will produce zygotic monoembryonic seeds that will not Naartje come true to type.