University of Dundee DOCTOR of PHILOSOPHY Marine Invasive
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trends of Aquatic Alien Species Invasions in Ukraine
Aquatic Invasions (2007) Volume 2, Issue 3: 215-242 doi: http://dx.doi.org/10.3391/ai.2007.2.3.8 Open Access © 2007 The Author(s) Journal compilation © 2007 REABIC Research Article Trends of aquatic alien species invasions in Ukraine Boris Alexandrov1*, Alexandr Boltachev2, Taras Kharchenko3, Artiom Lyashenko3, Mikhail Son1, Piotr Tsarenko4 and Valeriy Zhukinsky3 1Odessa Branch, Institute of Biology of the Southern Seas, National Academy of Sciences of Ukraine (NASU); 37, Pushkinska St, 65125 Odessa, Ukraine 2Institute of Biology of the Southern Seas NASU; 2, Nakhimova avenue, 99011 Sevastopol, Ukraine 3Institute of Hydrobiology NASU; 12, Geroyiv Stalingrada avenue, 04210 Kiyv, Ukraine 4Institute of Botany NASU; 2, Tereschenkivska St, 01601 Kiyv, Ukraine E-mail: [email protected] (BA), [email protected] (AB), [email protected] (TK, AL), [email protected] (PT) *Corresponding author Received: 13 November 2006 / Accepted: 2 August 2007 Abstract This review is a first attempt to summarize data on the records and distribution of 240 alien species in fresh water, brackish water and marine water areas of Ukraine, from unicellular algae up to fish. A checklist of alien species with their taxonomy, synonymy and with a complete bibliography of their first records is presented. Analysis of the main trends of alien species introduction, present ecological status, origin and pathways is considered. Key words: alien species, ballast water, Black Sea, distribution, invasion, Sea of Azov introduction of plants and animals to new areas Introduction increased over the ages. From the beginning of the 19th century, due to The range of organisms of different taxonomic rising technical progress, the influence of man groups varies with time, which can be attributed on nature has increased in geometrical to general processes of phylogenesis, to changes progression, gradually becoming comparable in in the contours of land and sea, forest and dimensions to climate impact. -

Annelida: Polychaeta) in a Marine Cave of the Ionian Sea (Italy, Central Mediterranean)
J. Mar. Biol. Ass. U.K. (2006), 86,1373^1380 doi: 10.1017/S0025315406014408 Printed in the United Kingdom Recruitment of Serpuloidea (Annelida: Polychaeta) in a marine cave of the Ionian Sea (Italy, central Mediterranean) P O Francesco Denitto* and Margherita Licciano *Laboratorio di Zoogeogra¢a e Faunistica, DiSTeBA, Universita' di Lecce, Via Prov.le Lecce-Monteroni, 73100 Lecce, Italy. O Laboratorio di Zoologia e Simbiosi, DiSTeBA, Universita' di Lecce, Via Prov.le Lecce-Monteroni, 73100 Lecce, Italy. P Corresponding author, e-mail: [email protected] This paper is the ¢rst attempt to study the recruitment of Serpuloideans in a Mediterranean marine cave through the use of arti¢cial substrates placed in three di¡erent positions, from the entrance to the interior of the cave. This study provides qualitative and quantitative data concerning Serpuloidea recruitment on panels removed successively after one, three, six and 12 months of permanence in the cave. A homogeneous distribution of juveniles Spirorbidae throughout the cave axis had already been detected after one month of panel immersion. Spirorbids were recorded also on panels removed after three months as well as serpulids, which began to be detected during this sampling time, even though represented by just one species. A signi¢cantly di¡erent pattern of distribution throughout the cave axis was observed after only six months, while other serpulids were detected for the ¢rst time. The pattern of species distribution seemed to re£ect the biotic and environmental conditions of the cave. The highest serpuloidean species abundance and diversity was found on panels placed in the intermediate position within the cave. -

Polychaeta: Serpulidae) from Southeastern Australia
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Knight-Jones, E. W., P. Knight-Jones and L. C. Llewellyn, 1974. Spirorbinae (Polychaeta: Serpulidae) from southeastern Australia. Notes on their taxonomy, ecology, and distribution. Records of the Australian Museum 29(3): 106–151. [1 May 1974]. doi:10.3853/j.0067-1975.29.1974.230 ISSN 0067-1975 Published by the Australian Museum, Sydney naturenature cultureculture discover discover AustralianAustralian Museum Museum science science is is freely freely accessible accessible online online at at www.australianmuseum.net.au/publications/www.australianmuseum.net.au/publications/ 66 CollegeCollege Street,Street, SydneySydney NSWNSW 2010,2010, AustraliaAustralia o ~------------------~o 25-20 and above 23-17 19- 14 17- 13 ood below 20 20 40 40 ,-- 50 50 Figure I.-Top left, collecting sites near Adelaide; top right, ditto near Sydney; bottom, collecting locations inset, showing also currents of warm, cool (interrupted lines) and cold water (dotted lines). A second smaller arrow-head indicates that the current occasionally reverses. Coastal water types and the mean position of the subtropical convergence (see p. 147) modified from Knox (1963), with mean summer (February) and winter (August) temperatures in degreesC. SPIRORBINAE (POLYCHAETA: SERPULIDAE) FROM SOUTHEASTERN AUSTRALIA. Notes on their Taxonomy, Ecology, and Distribution By E. W. KNIGHT-jONES and PHYLLIS KNIGHT-jONES University College of Swansea, U.K. and L. C. LLEWELLYN, New South Wales State Fisheries, Sydney, Australia Figures 1-14 Manuscript received, 1st September, 1972 SUMMARY Fifteen species belonging to seven genera are described, with pictorial and dichotomous keys to identification and notes on their distribution in other regions. All occur on or adjoining the shore, or on seaweeds cast ashore. -

Across-Shelf Larval, Postlarval, and Juvenile Fish Collected at Offshore Oil and Gas Platforms and a Coastal Rock Jetty West of the Mississippi River Delta
OCS Study MMS 2001-077 Coastal Marine Institute Across-Shelf Larval, Postlarval, and Juvenile Fish Collected at Offshore Oil and Gas Platforms and a Coastal Rock Jetty West of the Mississippi River Delta U .S . Department of the Interior AnK Cooperative Agreement Minerals 11Aanagement Service Coastal Marine Institute Adw Gulf of Mexico OCS Region Louisiana State University IR OCS Study MMS 2001-077 Coastal Marine Institute Across-Shelf Larval, Postlarval, and Juvenile Fish Collected at Offshore Oil and Gas Platforms and a Coastal Rock Jetty West of the Mississippi River Delta Authors Frank J. Hernandez, Jr. Richard F. Shaw Joseph S . Cope James G . Ditty Mark C. Benfield Talat Farooqi September 2001 Prepared under MMS Contract 14-35-0001-30660-19926 by Coastal Fisheries Institute Louisiana State University Baton Rouge, Louisiana 70803 Published by U .S. Department of the Interior Cooperative Agreement Minerals Management Service Coastal Marine Institute Gulf of Mexico OCS Region Louisiana State University DISCLAIMER This report was prepared under contract between the Minerals Management Service (MMS) and the Coastal Fisheries Institute (CFI), Louisiana State University (LSU). This report has been technically reviewed by the MMS and it has been approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of LSU or the MMS, nor does mention of trades names or commercial products constitute endorsement or recommendation for use. It is, however, exempt from review and compliance with the MMS editorial standard. REPORT AVAILABILITY Extra copies of the report may be obtained from the Public Information Office (Mail Stop 5034) at the following address : U.S . -

Change in Tropical Rocky Shore Communities Due to an Alien Coral Invasion
Vol. 438: 85–96, 2011 MARINE ECOLOGY PROGRESS SERIES Published October 5 doi: 10.3354/meps09290 Mar Ecol Prog Ser Change in tropical rocky shore communities due to an alien coral invasion B. G. Lages1, B. G. Fleury2, C. Menegola3, J. C. Creed2,* 1Programa de Pós-Graduação em Ecologia e Evolução and 2Departamento de Ecologia, Instituto de Biologia Roberto Alcântara Gomes, Universidade do Estado do Rio de Janeiro — UERJ, CEP 20559-900 Rio de Janeiro, RJ, Brasil 3Departamento de Zoologia, Instituto de Biologia, Universidade Federal da Bahia, CEP 40170-290 Salvador, BA, Brasil ABSTRACT: To determine how benthic, tropical, rocky shore communities were affected by the invasive coral species Tubastraea coccinea and T. tagusensis, 8 sites were studied during 2 yr on rocky shores in the southwest Atlantic Ocean (Brazil) by using both fixed and random sampling techniques. Overall, mean cover of T. tagusensis was 0.7% and T. coccinea was 0.4%, (the eleventh and sixteenth most abundant taxa, respectively, throughout the sites). Forty-two major space occupying taxa were registered. In fixed quadrats there was a 76.6% increase per year in density of Tubastraea spp. over the study period. For percent cover no significant difference in cover over time was detected for T. coccinea, but for T. tagusensis and overall (both species) cover increased significantly. The random quadrats data showed subtle differences from the fixed quadrats. There was an increase in density of Tubastraea spp. through time (67.8% per year over the study period). In random samples the density of T. coccinea increased during the study but that of T. -

Sponge Community Structure and Anti-Predator Defenses on Temperate Reefs of the South Atlantic Bight
Georgia Southern University Digital Commons@Georgia Southern Electronic Theses and Dissertations Graduate Studies, Jack N. Averitt College of Fall 2005 Sponge Community Structure and Anti-Predator Defenses on Temperate Reefs of the South Atlantic Bight Richard Robert Ruzicka Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/etd Recommended Citation Ruzicka, Richard Robert, "Sponge Community Structure and Anti-Predator Defenses on Temperate Reefs of the South Atlantic Bight" (2005). Electronic Theses and Dissertations. 705. https://digitalcommons.georgiasouthern.edu/etd/705 This thesis (open access) is brought to you for free and open access by the Graduate Studies, Jack N. Averitt College of at Digital Commons@Georgia Southern. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. SPONGE COMMUNITY STRUCTURE AND ANTI-PREDATOR DEFENSES ON TEMPERATES REEFS OF THE SOUTH ATLANTIC BIGHT by RICHARD ROBERT RUZICKA III Under the Direction of Daniel F. Gleason ABSTRACT The interaction between predation and anti-predator defenses of prey is important in shaping community structure in all ecosystems. This study examined the relationship between sponge predation and the distribution of sponge anti-predator defenses on temperate reefs in the South Atlantic Bight. Significant differences in the distribution of sponge species, sponge densities, and densities of sponge predators were documented across two adjacent reef habitats. Significant differences also occurred in the distribution of sponge chemical and structural defenses with chemical deterrence significantly greater in sponges associated with the habitat having higher predation intensity. Structural defenses, although effective in some instances, appear to be inadequate against spongivorous predators thereby restricting the distribution of sponge species lacking chemical defenses to habitats with lower predation intensity. -

Malacofauna Marina Del Parque Nacional “Los Caimanes”, Villa Clara, Cuba
Tesis de Diploma Malacofauna Marina del Parque Nacional “Los Caimanes”, Villa Clara, Cuba. Autora: Liliana Olga Quesada Pérez Junio, 2011 Universidad Central “Marta Abreu” de Las Villas Facultad Ciencias Agropecuaria TESIS DE DIPLOMA Malacofauna marina del Parque Nacional “Los Caimanes”, Villa Clara, Cuba. Autora: Liliana Olga Quesada Pérez Tutor: M. C. Ángel Quirós Espinosa Investigador Auxiliar y Profesor Auxiliar [email protected] Centro de Estudios y Servicios Ambientales, CITMA-Villa Clara Carretera Central 716, Santa Clara Consultante: Dr.C. José Espinosa Sáez Investigador Titular Instituto de Oceanología Junio, 2011 Pensamiento “La diferencia entre una mala observación y una buena, es que la primera es errónea y la segunda es incompleta.” Van Hise Dedicatoria Dedicatoria: A mis padres, a Yandy y a mi familia: por las innumerables razones que me dan para vivir, y por ser fuente de inspiración para mis metas. Agradecimientos Agradecimientos: Muchos son los que de alguna forma contribuyeron a la realización de este trabajo, todos saben cuánto les agradezco: Primero quiero agradecer a mis padres, que aunque no estén presentes sé que de una forma u otra siempre estuvieron allí para darme todo su amor y apoyo. A mi familia en general: a mi abuela, hermano, a mis tíos por toda su ayuda y comprensión. A Yandy y a su familia que han estado allí frente a mis dificultades. Agradecer a mi tutor el M.Sc. Ángel Quirós, a mi consultante el Dr.C. José Espinosa y a la Dra.C. María Elena, por su dedicación para el logro de esta tesis. A mis compañeros de grupo por estos cinco años que hemos compartidos juntos, que para mí fueron inolvidables. -

On Some Hydroids (Cnidaria) from the Coast of Pakistan
Pakistan J. Zool., vol. 38(3), pp. 225-232, 2006. On Some Hydroids (Cnidaria) from the Coast of Pakistan NASEEM MOAZZAM AND MOHAMMAD MOAZZAM Institute of Marine Sciences, University of Karachi, Karachi 75270, Pakistan (NM) and Marine Fisheries Department, Government of Pakistan, Fish Harbour, West Wharf, Karachi 74900, Pakistan (MM) Abstract .- The paper deals with the occurrence of eleven species of the hydroids from the coast of Pakistan. All the species are reported for the first time from Pakistan. These species are Hydractinia epidocleensis, Pennaria disticha, Eudendrium capillare, Orthopyxis cf. crenata, Clytia noliformis, C. hummelincki, Dynamena crisioides, D. quadridentata, Sertularia distans, Pycnotheca mirabilis and Macrorhynchia philippina. Key words: Hydroids, Coelenterata, Pakistan, Hydractinia, Pennaria, Eudendrium, Orthopyxis, Clytia, Dynamena, Sertularia, Pycnotheca, Macrorhynchia. INTRODUCTION used in the paper are derived from Millard (1975), Gibbons and Ryland (1989), Ryland and Gibbons (1991). In comparison to other invertebrates, TAXONOMIC ENUMERATION hydroids are one of the least known groups of marine animals from the coast of Pakistan Haque Family BOUGAINVILLIIDAE (1977) reported a few Cnidaria from the Pakistani Genus HYDRACTINIA Van Beneden, 1841 coast including two hydroids i.e. Plumularia flabellum Allman, 1883 (= P. insignis Allman, 1. Hydractinia epidocleensis Leloup, 1931 1883) and Campanularia juncea Allman, 1874 (= (Fig. 1) Thyroscyphus junceus (Allman, 1876) from Keamari and Bhit Island, Karachi, respectively. Ahmed and Hameed (1999), Ahmed et al. (1978) and Haq et al. (1978) have mentioned the presence of hydroids in various habitats along the coast of Pakistan. Javed and Mustaquim (1995) reported Sertularia turbinata (Lamouroux, 1816) from Manora Channel, Karachi. The present paper describes eleven species of Cnidaria collected from the Pakistani coast all of which are new records for Pakistan. -

Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2016 Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes Christi Linardich Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biodiversity Commons, Biology Commons, Environmental Health and Protection Commons, and the Marine Biology Commons Recommended Citation Linardich, Christi. "Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes" (2016). Master of Science (MS), Thesis, Biological Sciences, Old Dominion University, DOI: 10.25777/hydh-jp82 https://digitalcommons.odu.edu/biology_etds/13 This Thesis is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES by Christi Linardich B.A. December 2006, Florida Gulf Coast University A Thesis Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE BIOLOGY OLD DOMINION UNIVERSITY August 2016 Approved by: Kent E. Carpenter (Advisor) Beth Polidoro (Member) Holly Gaff (Member) ABSTRACT HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES Christi Linardich Old Dominion University, 2016 Advisor: Dr. Kent E. Carpenter Understanding the status of species is important for allocation of resources to redress biodiversity loss. -

New Records of Commercially Valuable Black Corals (Cnidaria: Antipatharia) from the Northwestern Hawaiian Islands at Mesophotic Depths1
New Records of Commercially Valuable Black Corals (Cnidaria: Antipatharia) from the Northwestern Hawaiian Islands at Mesophotic Depths1 Daniel Wagner,2,8 Yannis P. Papastamatiou,3 Randall K. Kosaki,4 Kelly A. Gleason,4 Greg B. McFall,5 Raymond C. Boland,6 Richard L. Pyle,7 and Robert J. Toonen2 Abstract: Mesophotic coral reef ecosystems are notoriously undersurveyed worldwide and particularly in remote locations like the Northwestern Hawaiian Islands ( NWHI). A total of 37 mixedgas technical dives were performed to depths of 80 m across the NWHI to survey for the presence of the invasive oc tocoral Carijoa sp., the invasive red alga Acanthophora spicifera, and conspicuous megabenthic fauna such as black corals. The two invasive species were not re corded from any of the surveys, but two commercially valuable black coral spe cies, Antipathes griggi and Myriopathes ulex, were found, representing substantial range expansions for these species. Antipathes griggi was recorded from the is lands of Necker and Laysan in 58 – 70 m, and Myriopathes ulex was recorded from Necker Island and Pearl and Hermes Atoll in 58 – 70 m. Despite over 30 yr of research in the NWHI, these black coral species had remained undetected. The new records of these conspicuous marine species highlight the utility of deep diving technologies in surveying the largest part of the depth range of coral reef ecosystems (40 – 150 m), which remains largely unexplored. The Papahänaumokuäkea Marine Na western Hawaiian Islands ( NWHI) repre tional Monument surrounding the North sents the largest marine protected area under U.S. jurisdiction, encompassing over 351,000 km2 and spanning close to 2,000 km from 1 This work was funded in part by the Western Pa Nïhoa Island to Kure Atoll. -
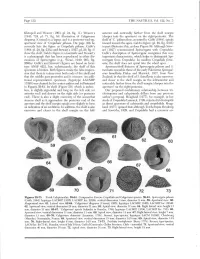
(1943: 724, Pi. 71, Fig. 16) Illustration of Calyptraea (Deeper Into the Aperture) on the Right/Posterior
Page 118 THE NAUTILUS, Vol. 122, No. 3 Kleinpeil and Weaver (1963, pi. 24, fig. 11). Weavers anterior and noticeably farther from the shell margin (1943: 724, pi. 71, fig. 16) illustration of Calyptraea (deeper into the aperture) on the right/posterior. The diegoana (Conrad) is a lapsus and is a posterior-end-up, shelf of 'C.y pileum thus, as noted by Gabb (1864), spirals apertural view of 'Crepidula' pileum. On page 356 he inward toward the apex. Gabb's figure (pi. 29, fig. 233b) correctly lists the figure as Crepidula pileum. Gabb's in part illustrates this, as does Figure 59. Although Stew- (1864: pi. 29, fig. 233a) and Stewart's (1927, pi. 29, fig. 3) art (1927) synonomized Spirocrypta with Crepidula, show the shelf. Gabb's figure is a fascimile and Stewart's Gabb's description of Spirocrypta recognizes this very is a photograph that has been reproduced in other dis- important characteristic, which helps to distinguish Spi- cussions of Spirocrypta (e.g., Wenz, 1940: 903, fig. rocrypta from Crepidula. In modern Crepidula forni- 2660a). Gabb's and Stewart's figures are based on lecto- cata, the shelf does not spiral into the whorl apex. type ANSP 4221, but, unfortunately, the shelf of this Aperture/shelf features of Spirocrypta pileum and S. specimen is broken. Both figures create the false impres- inomata resemble those of the early Paleocene Spirogal- sion that there is a sinus near both ends of the shelf and erus lamellaria Finlay and Marwick, 1937, from New that the middle part protrudes and is concave. -

Alien Species in the Mediterranean Sea by 2010
Mediterranean Marine Science Review Article Indexed in WoS (Web of Science, ISI Thomson) The journal is available on line at http://www.medit-mar-sc.net Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution A. ZENETOS 1, S. GOFAS 2, M. VERLAQUE 3, M.E. INAR 4, J.E. GARCI’A RASO 5, C.N. BIANCHI 6, C. MORRI 6, E. AZZURRO 7, M. BILECENOGLU 8, C. FROGLIA 9, I. SIOKOU 10 , D. VIOLANTI 11 , A. SFRISO 12 , G. SAN MART N 13 , A. GIANGRANDE 14 , T. KATA AN 4, E. BALLESTEROS 15 , A. RAMOS-ESPLA ’16 , F. MASTROTOTARO 17 , O. OCA A 18 , A. ZINGONE 19 , M.C. GAMBI 19 and N. STREFTARIS 10 1 Institute of Marine Biological Resources, Hellenic Centre for Marine Research, P.O. Box 712, 19013 Anavissos, Hellas 2 Departamento de Biologia Animal, Facultad de Ciencias, Universidad de Ma ’laga, E-29071 Ma ’laga, Spain 3 UMR 6540, DIMAR, COM, CNRS, Université de la Méditerranée, France 4 Ege University, Faculty of Fisheries, Department of Hydrobiology, 35100 Bornova, Izmir, Turkey 5 Departamento de Biologia Animal, Facultad de Ciencias, Universidad de Ma ’laga, E-29071 Ma ’laga, Spain 6 DipTeRis (Dipartimento per lo studio del Territorio e della sue Risorse), University of Genoa, Corso Europa 26, 16132 Genova, Italy 7 Institut de Ciències del Mar (CSIC) Passeig Mar tim de la Barceloneta, 37-49, E-08003 Barcelona, Spain 8 Adnan Menderes University, Faculty of Arts & Sciences, Department of Biology, 09010 Aydin, Turkey 9 c\o CNR-ISMAR, Sede Ancona, Largo Fiera della Pesca, 60125 Ancona, Italy 10 Institute of Oceanography, Hellenic Centre for Marine Research, P.O.