Mineralogical and Geochemical Profiling of Arsenic-Contaminated Alluvial Aquifers in the Ganges-Brahmaputra Floodplain Manikg
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Initial Environmental Examination
Bangladesh Power System Enhancement and Efficiency Improvement Project (RRP BAN 49423) Initial Environmental Examination March 2017 Bangladesh: Bangladesh Power System Enhancement and Efficiency Improvement Project Prepared by Power Grid Company of Bangladesh Limited (PGCBL) and Bangladesh Rural Electrification Board (BREB), Government of Bangladesh for the Asian Development Bank. This initial environmental examination is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. Your attention is directed to the “terms of use” section on ADB’s website. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area.: Initial Environmental Examination Bangladesh: Bangladesh Power System Enhancement and Efficiency Improvement Project (Component 1: Transmission System Development in Southern Bangladesh) Prepared by Power Grid Company of Bangladesh Limited (PGCBL), Government of Bangladesh for the Asian Development Bank. CURRENCY EQUIVALENTS (as of 22 September 2016) Currency unit – Taka (Tk) Tk.1.00 = USD0.01276 USD 1.00 = Tk. 78.325 This initial environmental examination is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. Your attention is directed to the “terms of use” section on ADB’s website. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. -

INTERNATIONAL JOURNAL of ENVIRONMENT Volume-10, Issue-1, 2020/21 ISSN 2091-2854 Received:3 Dec 2020 Revised:24 Feb 2021 Accepted:26 Feb 2021
INTERNATIONAL JOURNAL OF ENVIRONMENT Volume-10, Issue-1, 2020/21 ISSN 2091-2854 Received:3 Dec 2020 Revised:24 Feb 2021 Accepted:26 Feb 2021 EVALUATION OF CONTAMINATION AND ACCUMULATION OF HEAVY METALS IN THE DHALESWARI RIVER SEDIMENTS, BANGLADESH Abdullah Al Mamun1, †, Protima Sarker 1, 2,*, †, Md. Shiblur Rahaman1, 3, Mohammad Mahbub Kabir1, 4 and Masahiro Maruo2 1Department of Environmental Science and Disaster Management, Noakhali Science and Technology University, Noakhali-3814, Bangladesh. 2School of Environmental Science, University of Shiga Prefecture, 2500 Hassakacho, Hikone, Shiga 522-8533, Japan. 3Department of Environmental and Preventive Medicine, Jichi Medical University School of Medicine, 3311-1 Yakushiji, Shimotsuke, Tochigi-329-0498, Japan. 4Research Cell, Noakhali Science and Technology University, Noakhali-3814, Bangladesh. *Corresponding author: [email protected] †Authors contributed equally to the manuscript Abstract The Dhaleswari river is considered as one of the most important rivers of Bangladesh due to its geographical location and ecological services. The present study attempts to evaluate the degree of heavy metal pollution, contamination, and accumulative behavior in the sediment of the Dhaleswari river. The sediment samples were collected from fifteen different locations of the Dhaleswari river. Heavy metals were analyzed using the Flame Atomic Spectrophotometer (FAAS). The mean concentrations of Zn, Cu, Cr, Pb and Cd were 131.9, 48.89, 43.16, 33.23 and 0.37 mgkg-1, respectively. According to the United States Environmental Protection Agency (USEPA) Sediment Quality Guideline, the sediment of most of the locations were not polluted for Pb and Cd. But S-11 location for Cd (0.8 mg kg-1) was highly polluted. -

12. FORMULATION of the URBAN TRANSPORT MASTER PLAN Development of the RSTP Urban Transportation Master Plan (1) Methodology
The Project on The Revision and Updating of the Strategic Transport Plan for Dhaka (RSTP) Final Report 12. FORMULATION OF THE URBAN TRANSPORT MASTER PLAN Development of the RSTP Urban Transportation Master Plan (1) Methodology The development of the RSTP Urban Transportation Master Plan adopted the following methodology (see Figure 12.1): (i) Elaborate the master plan network through a screen line analysis by comparing the network capacity and future demand. (ii) Identify necessary projects to meet future demand at the same time avoiding excessive capacity. (iii) Conducts economic evaluation of each project to give priority on projects with higher economic return. (iv) Conduct preliminary environmental assessment of every project and consider countermeasures against environmental problems, if any. (v) Make a final prioritization of all physical projects by examining their respective characteristics from different perspectives. (vi) Classify the projects into three categories, namely short-, medium- and long-term projects, by considering the financial constraints. (vii) Prepare an action plan for short-term projects together with “soft” measures. Mid-term Project Source: RSTP Study Team Figure 12.1 Development Procedure for the Master Plan 12-1 The Project on The Revision and Updating of the Strategic Transport Plan for Dhaka (RSTP) Final Report (2) Output of the Transportation Network Plan The RSTP urban transportation network plan was developed based on a review and a modification of the STP network plan. The main points of the modification or adoption of the STP network master plan are as follows: i. Harmonization with future urban structure, land-use plan and development of network plan. -

Connecting Bangladesh: Economic Corridor Network
Connecting Bangladesh: Economic Corridor Network Economic corridors are anchored on transport corridors, and international experience suggests that the higher the level of connectivity within and across countries, the higher the level of economic growth. In this paper, a new set of corridors is being proposed for Bangladesh—a nine-corridor comprehensive integrated multimodal economic corridor network resembling the London Tube map. This paper presents the initial results of the research undertaken as an early step of that development effort. It recommends an integrated approach to developing economic corridors in Bangladesh that would provide a strong economic foundation for the construction of world-class infrastructure that, in turn, could support the growth of local enterprises and attract foreign investment. About the Asian Development Bank COnnecTING BANGLADESH: ADB’s vision is an Asia and Pacific region free of poverty. Its mission is to help its developing member countries reduce poverty and improve the quality of life of their people. Despite the region’s many successes, it remains home to a large share of the world’s poor. ADB is committed to reducing poverty through inclusive economic growth, environmentally sustainable growth, and regional integration. ECONOMIC CORRIDOR Based in Manila, ADB is owned by 67 members, including 48 from the region. Its main instruments for helping its developing member countries are policy dialogue, loans, equity investments, guarantees, grants, NETWORK and technical assistance. Mohuiddin Alamgir -

July 2016 Volume 3 No 3
6 VOLUME 3 NO 3 JULY 2016 DHAKA CENTRAL INTERNATIONAL MEDICAL COLLEGE JOURNAL (APPROVED BY BMDC) July 2016, Vol. 3 No. 3 Contents From the Desk of Editor-in-Chief 3 Instructions for Authors 4 Editorial Novel Treatment of Diabetic Nephropathy 12 Original Articles Incidence of Malignancy in Thyroid Nodule 14 Abedin SAMA, Alam MM, Islam MS, Fakir MAY Dyslipidemia and Atherogenic Index among the 21 Young Female Doctors ofBangladesh. Khanduker S, Hoque MM, Khanduker N, Chowdhury MAA, Nazneen M A Study on Stroke in Young Patients due to Cardiac 26 Disease in a Tertiary Care Hospital in Dhaka City Mukta M, Mohammad QD, Mir AS Variation of Transverse Diameter ofDry Ossified 33 Human Atlas Vertebra of Male and Female Rahman S, Ara S, Sayeed S, Rashid S, Ferdous Z, Kashem K Study on Health Effects of Teenage Pregnancies among the Patients 36 Attending Antenatal Care Centre of Chittagong Medical College Hospital Tarafdar MA, Begum N, Das SR, Begum S, Sultana A, Rahman R, Begum R Identification ofDifferent Clinical Features and Complications of 41 Type 2 Diabetes Mellitus in Bengaladeshi Males Begum F, Shamim KM, Akter S, Hossain S, Nazma N, Afrin M, Moureen A Review Articles Female Genital Tuberculosis- A Review Article 46 Shaheed S, Mamun SMAA, Khanom M Case Reports Round Worm induced Acute Appendicitis- an Incidental 51 Finding during Colonoscopy Masum QAA, Islam MN 1 Dhaka Central International Medical College Journal Vol.13 No. 3July 2016 An Official Organ of Dhaka Central International Medical College CHIEF PATRON ADVISORS The Dhaka Central International Prof. Md. Anwarul Islam Md. -

Everyday Forms of Collective Action in Bangladesh
CAPRi Working Paper No. 94 January 2009 EVERYDAY FORMS OF COLLECTIVE ACTION IN BANGLADESH Learning from Fifteen Cases Peter Davis, University of Bath with Rafiqul Haque, Data Analysis and Technical Assistance (DATA), Bangladesh Dilara Hasin, DATA Md. Abdul Aziz, DATA Anowara Begum, DATA CGIAR Systemwide Program on Collective Action and Property Rights (CAPRi) C/– International Food Policy Research Institute, 2033 K Street NW, Washington, DC 20006–1002 USA T +1 202.862.5600 • F +1 202.467.4439 • www.capri.cgiar.org The CGIAR Systemwide Program on Collective Action and Property Rights (CAPRi) is an initiative of the 15 centers of the Consultative Group on International Agricultural Research (CGIAR). The initiative promotes comparative research on the role of property rights and collective action institutions in shaping the efficiency, sustainability, and equity of natural resource systems. CAPRi’s Secretariat is hosted within the Environment and Production Technology Division (EPTD) of the International Food Policy Research Institute (IFPRI). CAPRi receives support from the Governments of Norway, Italy and the World Bank. CAPRi Working Papers contain preliminary material and research results. They are circulated prior to a full peer review to stimulate discussion and critical comment. It is expected that most working papers will eventually be published in some other form and that their content may also be revised. Cite as: Davis, P. 2009. Everyday Forms of Collective Action in Bangladesh: Learning from Fifteen Cases. CAPRi Working Paper No. 94. International Food Policy Research Institute: Washington, DC. http://dx.doi.org/10.2499/CAPRiWP94. Copyright © January 2009. International Food Policy Research Institute. All rights reserved. -

Manikganj Manikganj Is a District Located in Central Bangladesh
Manikganj Manikganj is a district located in central Bangladesh. It is a part of the Dhaka division, with an area of 1,379 square kilometres. It is one of the nearest districts to Dhaka, only 70 kilometres away from the city. It bound by the Tangail district in the north, Dhaka district in the east, Faridpur district in the south, the Padma and Jamuna rivers Photo credit: BRAC and the districts of Pabna and Rajbari in the west. The main Ayesha Abed Foundation was started in 1978 as part of BRAC’s development interventions to organise, train and support rural women through traditional handicrafts rivers are the Padma, Jamuna, Dhaleshwari, Ichamati and General information Targeting the ultra poor Kaliganga. Specially targeted ultra Population 1,440,000 poor (STUP) members 450 This city is surrounded by rivers. As Unions 65 Others targeted ultra a result few of the sub-districts are Villages 1,873 poor (OTUP) members 725 affected by river bank erosion every Children (0-15) 860,000 Asset received 450 year. The people of Manikganj Training received 725 Primary schools 607 are mostly involved in agriculture. Healthcare availed 175 BRAC started its operation here Literacy rate 56% in 1974. Right now, most of Hospitals 7 Education BRAC’s core programmes, such NGOs 83 Primary schools 63 Banks 35 as microfinance, education (BEP), Pre-primary schools 225 health, nutrition and population Bazaars 98 Adolescent development (HNPP), targeting the ultra poor programme (ADP) centres 298 (TUP), community empowerment Community libraries (CEP), migration and human rights At a glance (gonokendros) 53 and legal aid services (HRLS). -
UNC Parking Zone Map UNC Transportation & Parking
UNC Parking Zone Map UNC Transportation & Parking Q R S T U V W X Y Z A B C D E F G H I J K L M N O P 26 **UNC LEASES SPACE CAROLINA . ROAD IN THESE BUILDINGS 21 21 MT HOMESTEAD NORTH LAND MGMT. PINEY OPERATIONS CTR. VD. (NC OFFICE HORACE WILLIAMS AIRPORT VD., HILL , JR. BL “RR” 41 1 1 Resident 41 CommuterRR Lot R12 UNC VD AND CHAPEL (XEROX) TE 40 MLK BL A PRINTING RIVE EXTENSION MLK BL ESTES D SERVICES TIN LUTHER KING TERST PLANT N O I AHEC T EHS HOMESTEAD ROAD MAR HANGER VD. 86) O I-40 STORAGE T R11 TH (SEE OTHER MAPS) 22 22 O 720, 725, & 730 MLK, JR. BL R1 T PHYSICAL NOR NORTH STREET ENVRNMEN HL .3 MILES TO TH. & SAFETY ESTES DRIVE 42 COMMUTER LOT T. 42 ER NC86 ELECTRICAL DISTRICENTBUTION OPERATIONS SURPLUS WA REHOUSE N1 ST GENERAL OREROOM 2 23 23 2 R1 CHAPEL HILL ES MLK JR. BOULE NORTH R1 ARKING ARD ILITI R1 / R2OVERFLOW ZONEP V VICES C R A F SHOPS GY SE EY 43 RN 43 ENERBUILDING CONSTRUCTION PRITCHARD STREET R1 NC 86 CHURCH STREET . HO , JR. BOULE ES F R1 / V STREET SER L BUILDING VICE ARD A ST ATIO GI EET N TR AIRPOR R2 S T DRIVE IN LUTHER KING BRANCH T L MAR HIL TH WEST ROSEMARY STREET EAST ROSEMARY STREET L R ACILITIES DRIVE F A NO 24 STUDRT 24 TH COLUMBI IO CHAPE R ADMINIST OFF R NO BUILDINGICE ATIVE R10 1700 N9 MLK 208 WEST 3 N10 FRANKLIN ST. -
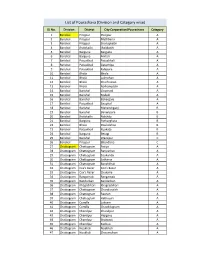
List of Pourashava (Division and Category Wise)
List of Pourashava (Division and Category wise) SL No. Division District City Corporation/Pourashava Category 1 Barishal Pirojpur Pirojpur A 2 Barishal Pirojpur Mathbaria A 3 Barishal Pirojpur Shorupkathi A 4 Barishal Jhalokathi Jhalakathi A 5 Barishal Barguna Barguna A 6 Barishal Barguna Amtali A 7 Barishal Patuakhali Patuakhali A 8 Barishal Patuakhali Galachipa A 9 Barishal Patuakhali Kalapara A 10 Barishal Bhola Bhola A 11 Barishal Bhola Lalmohan A 12 Barishal Bhola Charfession A 13 Barishal Bhola Borhanuddin A 14 Barishal Barishal Gournadi A 15 Barishal Barishal Muladi A 16 Barishal Barishal Bakerganj A 17 Barishal Patuakhali Bauphal A 18 Barishal Barishal Mehendiganj B 19 Barishal Barishal Banaripara B 20 Barishal Jhalokathi Nalchity B 21 Barishal Barguna Patharghata B 22 Barishal Bhola Doulatkhan B 23 Barishal Patuakhali Kuakata B 24 Barishal Barguna Betagi B 25 Barishal Barishal Wazirpur C 26 Barishal Pirojpur Bhandaria C 27 Chattogram Chattogram Patiya A 28 Chattogram Chattogram Bariyarhat A 29 Chattogram Chattogram Sitakunda A 30 Chattogram Chattogram Satkania A 31 Chattogram Chattogram Banshkhali A 32 Chattogram Cox's Bazar Cox’s Bazar A 33 Chattogram Cox's Bazar Chakaria A 34 Chattogram Rangamati Rangamati A 35 Chattogram Bandarban Bandarban A 36 Chattogram Khagrchhari Khagrachhari A 37 Chattogram Chattogram Chandanaish A 38 Chattogram Chattogram Raozan A 39 Chattogram Chattogram Hathazari A 40 Chattogram Cumilla Laksam A 41 Chattogram Cumilla Chauddagram A 42 Chattogram Chandpur Chandpur A 43 Chattogram Chandpur Hajiganj A -

Study on Surface Water Availability for Future Water Demand for Dhaka City
STUDY ON SURFACE WATER AVAILABILITY FOR FUTURE WATER DEMAND FOR DHAKA CITY MD EHSANUL HAQUE DOCTOR OF PHILOSOPHY (WATER RESOURCES ENGINEERING) DEPARTMENT OF WATER RESOURCES ENGINEERING BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY DHAKA, BANGLADESH FEBRUARY, 2018 STUDY ON SURFACE WATER AVAILABILITY FOR FUTURE WATER DEMAND FOR DHAKA CITY by Md Ehsanul Haque A thesis submitted to the Department of Water Resources Engineering Bangladesh University of Engineering and Technology, Dhaka in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY (WATER RESOURCES ENGINEERING) DEPARTMENT OF WATER RESOURCES ENGINEERING BANGLADESH UNIVERSITY OF ENGINEERING AND TECHNOLOGY DHAKA, BANGLADESH February, 2018 CERTIFICATE OF APPROVAL Signature of the Student Md Ehsanul Haque Signature of the Supervisor Professor Dr. Md. Abdul Matin iii ii To My Father Late Lt Col Shamsul Haque & My Mother Mrs Suraiya Haque iii ACKNOWLEDGEMENTS All praises are solely to the most merciful and beneficent Almighty Allah for enabling the author to complete the research work and to prepare this manuscript for fulfillment of the degree of Doctor of Philosophy in Water Resources Engineering. The author deems it is a great pleasure and honor to express his deep sense of gratitude, heartfelt indebtedness and sincere appreciation to his Thesis Supervisor Professor Dr. Md. Abdul. Matin, Department of Water Resources Engineering, Bangladesh University of Engineering and Technology for providing scholastic guidance, supervision and affectionate inspiration for successful achievement and outstanding contribution of the research work as well as preparation of this thesis. The author extends his sincere appreciation and immense indebtedness to his research to the distinguished members Professor Dr. -

Hydro-Morphological Assessment of the River Jamuna and Old Dhaleshwari Offtake
Proceedings of the 4th International Conference on Civil Engineering for Sustainable Development (ICCESD 2018), 9~11 February 2018, KUET, Khulna, Bangladesh (ISBN-978-984-34-3502-6) HYDRO-MORPHOLOGICAL ASSESSMENT OF THE RIVER JAMUNA AND OLD DHALESHWARI OFFTAKE Tasmiah Ahsan*1 and M. A. Matin2 1 Lecturer, Department of Civil Engineering, Stamford University, Bangladesh, e-mail: [email protected] 2 Professor, Department of WRE, BUET, Bangladesh, e-mail: [email protected] ABSTRACT The offtakes are important links between the main rivers and the distributaries. An example is the Jamuna and Old Dhaleshwari offtake. The mouth of the river at offtake is not stable. At present, serious deposition has taken place at the mouth. This paper presents the hydro-morphological analysis of the Jamuna and Old Dhaleshwari offtake of Bangladesh to predict its sustainability. The present study has been undertaken to assess the hydraulic behavior of the Old Dhaleshwari River based on its flow carrying capacity. Satellite images, old maps and hydro-morphological data have been used to understand the morphology and planform of the river. From conveyance analysis rating curves have been developed for the cross sections in the vicinity of offtake for both Jamuna and Old Dhaleshwari. Analysis of historical hydrometric data and satellite images near the offtake has been carried out. Keywords: River offtake, River morphology, Rating curve, Conveyance analysis 1. INTRODUCTION The Old Dhaleshwari River is a distributary, 160 km long, of the Jamuna River in central Bangladesh. It starts off the Jamuna near the northwestern tip of Tangail District (BWDB, 2011). The distribution of discharge and sediment transport at river offtake is a key factor for the long term morphological development of the main rivers (FAP24, 1996a). -

A Case Study of Manikganj Sadar Upazila
Journal of Geographic Information System, 2015, 7, 579-587 Published Online December 2015 in SciRes. http://www.scirp.org/journal/jgis http://dx.doi.org/10.4236/jgis.2015.76046 Dynamics of Land Use/Cover Change in Manikganj District, Bangladesh: A Case Study of Manikganj Sadar Upazila Marju Ben Sayed, Shigeko Haruyama Department of Environment Science and Technology, Mie University, Tsu, Japan Received 29 October 2015; accepted 1 December 2015; published 4 December 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract This study revealed land use/cover change of Manikganj Sadar Upazila concerning with urbaniza- tion of Dhaka city. The study area also offers better residential opportunity and food support for Dhaka city. The major focus of this study is to find out the spatial and temporal changes of land use/cover and its effects on urbanization while Dhaka city is an independent variable. For analyz- ing land use/cover change GIS and remote sensing technique were used. The maps showed that, between 1989 and 2009 built-up areas increased approximately +12%, while agricultural land decreased −7%, water bodies decreased about −2% and bare land decreased about −2%. The sig- nificant change in agriculture land use is observed in the south-eastern and north eastern site of the city because of nearest distance and better transportation facilities with Dhaka city. This study will contribute to the both the development of sustainable urban land use planning decisions and also for forecasting possible future changes in growth patterns.