Normal Imaging Findings of the Uterus 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Reproductive System
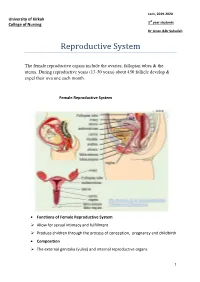
Lec1, 2019-2020 University of Kirkuk 3rd year students College of Nursing Dr Jenan &Dr Suhailah Reproductive System The female reproductive organs include the ovaries, fallopian tubes & the uterus. During reproductive years (13-50 years) about 450 follicle develop & expel their ova one each month. Female Reproductive System Functions of Female Reproductive System Allow for sexual intimacy and fulfillment Produce children through the process of conception, pregnancy and childbirth Composition The external genitalia (vulva) and internal reproductive organs 1 External genitalia of the female reproductive system: Mons pubis Labia majora Labia minora Clitoris Vestibule Perineum Internal Reproductive Organs Vagina (Birth canal) - A muscular tube that leads from the vulva to the uterus Uterus (Womb) A hollow, pear-shaped muscular structure Functions of the uterus: 1. Prepare for pregnancy each month 2. Protect and nourish the growing child Four sections: Cervix - Connect the vagina and uterus - Outer os Uterine Isthmus - Connects the cervix to the main body of the uterus - Thinnest portion of the uterus, and does not participate in the muscular Contractions of labor - Most likely to rupture during childbirth Corpus (Body) - Main body of the uterus 2 Fundus Topmost section of the uterus Walls of the corpus and fundus have three layers - Perimetrium - Myometrium - Endometrium Paired fallopian tubes - Tiny, muscular corridors 8-14 cm long 3 sections 1. Isthmus 2. Ampulla 3. Infundibulum Ovaries Two sex glands homologous to the male testes; located on either side of the uterus Functions: - Produce the female hormones estrogen and progesterone - Store ova and help them to mature - Regulate the menstrual cycle in response to anterior pituitary hormones 3 Regulation of Reproductive function: Puberty - The time of life in which an individual become capable of sexual reproduction. -

A Case of Non-Communicating Uterine Horn Containing Functional Endometrium
logy & Ob o st ec e tr n i y c s G Rani et al., Gynecol Obstet (Sunnyvale) 2015, 5:9 Gynecology & Obstetrics DOI: 10.4172/2161-0932.1000320 ISSN: 2161-0932 Case Report Open Access A Case of Non-Communicating Uterine Horn Containing Functional Endometrium Anjali Rani*, Madhu Kumari and Shipra Department of Obstetrics and Gynaecology, Institute Of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India Abstract Uterine anoamalies are very rare. They can present with complains of amebnorrhoea, dysmenorrhoea, bad obstetrical outcome and infertility etc. Unicornuate uterus with rudimentary horn is very rare. The incidence of this is 1/100,000. Normally rudimentary horns are non- functional and non-communicating. But if they have functional endometrium they can develop hematometra. We are presenting a case of hematometra and pain in a patient with rudimentary non communicating horn with functional endometrium. Rudimentary horn should be kept as a differential diagnosis in pelvic pain. Keywords: Unicornuate uterus; Rudimentary horn; Dysmenorrhea were found in the pelvis. Exploratory laparotomy was decided and the rudimentary horn was excised by applying clamps (Figure 2). Histo- Introduction pathological examination of the specimen was reported as uterine Uterine anomalies are very rarely seen. Unicornuate uterus is a very udimentary horn with functional endometrium with haematometra. rare uterine anomaly. The incidence of congenital uterine anomalies The patient was discharged on the fifth postoperative day. She came in in fertile population is 1/200 to 1/600. The incidence of rudimentary follow up her nomal menses after 6 weeks. horn is very very rare (1:100,000). -

A New Anatomic and Staging-Oriented Classification Of
cancers Perspective A New Anatomic and Staging-Oriented Classification of Radical Hysterectomy Mustafa Zelal Muallem Department of Gynecology with Center for Oncological Surgery, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Virchow Campus Clinic, Charité Medical University, 13353 Berlin, Germany; [email protected]; Tel.: +49-30-450-664373; Fax: +49-30-450-564900 Simple Summary: The main deficits of the available classifications of radical hysterectomy are the facts that they are based only on the lateral extension of resection, do not depend on the precise anatomy of parametrium and paracolpium and do not correlate with the tumour stage, size or infiltration in the vagina. This new suggested classification depends on the 3-dimentional concept of parametrium and paracolpium and the comprehensive description of the anatomy of parametrium, paracolpium and the pelvic autonomic nerve system. Each type in this classification tailored to the tumour stage according to FIGO- classification from 2018, taking into account the tumour size, localization and infiltration in the vaginal vault, which may make it the most suitable tool for planning and tailoring the surgery of radical hysterectomy. Abstract: The current understanding of radical hysterectomy more is centered on the uterus and little is being discussed about the resection of the vaginal cuff and the paracolpium as an essential part of this procedure. This is because that the current classifications of radical hysterectomy are based only on the lateral extent of resection. This way is easier to be understood but does not reflect Citation: Muallem, M.Z. -

Factors Associated with Parametrial Involvement
Original Article Obstet Gynecol Sci 2018;61(1):88-94 https://doi.org/10.5468/ogs.2018.61.1.88 pISSN 2287-8572 · eISSN 2287-8580 Factors associated with parametrial involvement in patients with stage IB1 cervical cancer: who is suitable for less radical surgery? Seung-Ho Lee1, Kyoung-Joo Cho1, Mi-Hyang Ko1, Hyun-Yee Cho2, Kwang-Beom Lee1, Soyi Lim1 1Departments of Obstetrics and Gynecology, 2Pathology, Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea Objective To detect the possible clinicopathologic factors associated with parametrial involvement in patients with stage IB1 cervical cancer and to identify a cohort of patients who may benefit from less radical surgery. Methods We retrospectively reviewed 120 patients who underwent radical hysterectomy and pelvic lymphadenectomy as treatment for stage IB1 cervical cancer. Results Overall, 18 (15.0%) patients had parametrial tumor involvement. Tumor size larger than 2 cm, invasion depth greater than 1 cm, presence of lymphovascular space involvement (LVSI), corpus involvement, and positive lymph nodes were statistically associated with parametrial involvement. Multivariate analysis for other factors showed invasion depth >1 cm (P=0.029), and corpus involvement (P=0.022) were significantly associated with parametrial involvement. A subgroup with tumor size smaller than 2 cm showed no parametrial involvement, regardless of invasion depth or presence of LVSI. Conclusion Tumor size smaller than 2 cm showed no parametrial involvement, regardless of invasion depth or presence of LVSI. Invasion depth >1 cm and corpus involvement were significantly associated with parametrial involvement in multivariate analysis. These finding may suggest that tumor size may a strong predictor of parametrial involvement in International Federation of Gynecology and Obstetrics stage IB1 cervical cancer, which can be used to select a subgroup population for less radical surgery. -

The Reproductive System
27 The Reproductive System PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The reproductive system is designed to perpetuate the species • The male produces gametes called sperm cells • The female produces gametes called ova • The joining of a sperm cell and an ovum is fertilization • Fertilization results in the formation of a zygote © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • Overview of the Male Reproductive System • Testis • Epididymis • Ductus deferens • Ejaculatory duct • Spongy urethra (penile urethra) • Seminal gland • Prostate gland • Bulbo-urethral gland © 2012 Pearson Education, Inc. Figure 27.1 The Male Reproductive System, Part I Pubic symphysis Ureter Urinary bladder Prostatic urethra Seminal gland Membranous urethra Rectum Corpus cavernosum Prostate gland Corpus spongiosum Spongy urethra Ejaculatory duct Ductus deferens Penis Bulbo-urethral gland Epididymis Anus Testis External urethral orifice Scrotum Sigmoid colon (cut) Rectum Internal urethral orifice Rectus abdominis Prostatic urethra Urinary bladder Prostate gland Pubic symphysis Bristle within ejaculatory duct Membranous urethra Penis Spongy urethra Spongy urethra within corpus spongiosum Bulbospongiosus muscle Corpus cavernosum Ductus deferens Epididymis Scrotum Testis © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • The Testes • Testes hang inside a pouch called the scrotum, which is on the outside of the body -

Clinical Pelvic Anatomy
SECTION ONE • Fundamentals 1 Clinical pelvic anatomy Introduction 1 Anatomical points for obstetric analgesia 3 Obstetric anatomy 1 Gynaecological anatomy 5 The pelvic organs during pregnancy 1 Anatomy of the lower urinary tract 13 the necks of the femora tends to compress the pelvis Introduction from the sides, reducing the transverse diameters of this part of the pelvis (Fig. 1.1). At an intermediate level, opposite A thorough understanding of pelvic anatomy is essential for the third segment of the sacrum, the canal retains a circular clinical practice. Not only does it facilitate an understanding cross-section. With this picture in mind, the ‘average’ of the process of labour, it also allows an appreciation of diameters of the pelvis at brim, cavity, and outlet levels can the mechanisms of sexual function and reproduction, and be readily understood (Table 1.1). establishes a background to the understanding of gynae- The distortions from a circular cross-section, however, cological pathology. Congenital abnormalities are discussed are very modest. If, in circumstances of malnutrition or in Chapter 3. metabolic bone disease, the consolidation of bone is impaired, more gross distortion of the pelvic shape is liable to occur, and labour is likely to involve mechanical difficulty. Obstetric anatomy This is termed cephalopelvic disproportion. The changing cross-sectional shape of the true pelvis at different levels The bony pelvis – transverse oval at the brim and anteroposterior oval at the outlet – usually determines a fundamental feature of The girdle of bones formed by the sacrum and the two labour, i.e. that the ovoid fetal head enters the brim with its innominate bones has several important functions (Fig. -

Left Vaginal Obstruction and Complex Left Uterine Horn Communication in a 12 Year Old Female Barry E
Perlman et al. Obstet Gynecol cases Rev 2015, 2:7 ISSN: 2377-9004 Obstetrics and Gynaecology Cases - Reviews Case Report: Open Access Left Vaginal Obstruction and Complex Left Uterine Horn Communication in a 12 Year Old Female Barry E. Perlman*, Amy S. Dhesi and Gerson Weiss Department of Obstetrics, Gynecology and Women’s Health, Rutgers - New Jersey Medical School, Newark, USA *Corresponding author: Barry E. Perlman DO, Department of Obstetrics, Gynecology and Women’s Health, Rutgers - New Jersey Medical School, MSB E-506, 185 South Orange Avenue, Newark, NJ 07101-1709, USA, Tel: 732 233 0997, E-mail: [email protected] Transabdominal pelvic sonogram revealed two prominent uterine Abstract cornua with an endometrial thickness of 3 mm in each horn. The Obstructive Müllerian duct anomalies are an infrequently right cornu measured 11.4 x 2.0 x 3.6 cm and the left cornu measured encountered clinical problem. The use of imaging and surgical 10.4 x 2.8 x 4.1 cm. A 7 cm mass in the endocervical canal, concerning exploration allowed for diagnosis and treatment of symptoms of a for hematocolpos, represented an occlusion extending to the left complex obstructive müllerian anomaly. We present a case of a 12 vagina (Figure 1). year old female with a history of intermittent lower abdominal pain and absent left kidney who was found to have an obstructed left She underwent further imaging with two MRI studies that were vagina and complex left uterine horn communications resulting in mutually inconclusive and inconsistent in regards to her pelvic hematocolpos, hematometra, and endometriosis. -

Clinical Research of Effects of Retaining the Uterine Blood Supply Hysterectomy on Ovarian Function
BIO Web of Conferences 8 , 01038 (2017)DOI: 10.1051/bioconf/201 70801038 ICMSB2016 Clinical research of effects of retaining the uterine blood supply hysterectomy on ovarian function Yufei Cai 1 and Hongxia Sun 2,a 1Obstetrics and Gynecology Department,Affiliated Hospital of Beihua University 2 Pharmacological Department, Pharmacy, Beihua University jilin jilin 132011, China Abstract. Objective To evaluate the effect of hysterectomy for reserving the uterine blood supply on ovarian endocrine function and on symptoms of menopausal transition. Methods Uterine benign lesions should be line the uterus times total resection in 100 patients were randomly divided into hysterectomy group of retaining uterus vascular supply group(research group,n=50) and traditional total hysterectomy group (the control group, n=50), comparing two groups in operation time, intraoperative bleeding ,postoperative fever and residual polyp, blood tests were taken to check the serum sex hormone levels change and clinical observation for perimenopausal symptoms before and postoperative three months, six months, one year and two years at the same time respectively. Results There was no significant difference between two groups in operation time, intraoperative blood loss, postoperative fever and residual polyp (P>0.05). There was no significant difference among research group before and after operation in serum sex hormones(P>0.05),the symptoms of the menopausal transition hardly appear; postoperative FSH, LH increased significantly in control group (P<0.05),E2 decrease (P<0.05), perimenopausal symptoms appeared more often. Conclusion The effect of uterus hysterectomy for retaining vascular supply on ovarian endocrine function is less than the traditional total hysterectomy, this operation has a certain importance to preserve ovarian function and delay the occurrence of premature ovarian aging. -

REPRODUCTIVE SYSTEM Vasco Dominic
REPRODUCTIVE SYSTEM Vasco Dominic ORGANISATION Reproductive organs which produce gametes and hormones. Reproductive tract consisting of ducts, store and transport gametes. Accessory glands and organs that secrete fluids into the ducts of the reproductive system or into other secretory ducts. Perineal structures associated with the reproductive system, collectively known as external genitalia. The male and female systems are functionally different. In the male the gonads are the testes that secrete androgens, principally testosterone and produce a half billion sperms per day. After storage the sperm travel along a lengthy duct and mixed with secretions of the glands to form semen. In the female the gonads are the ovaries which produce only one mature gamete per month. The oocyte travels via a short duct into the muscular uterus. THE MALE REPRODUCTIVE SYSTEM TESTES Each has the shape of a flattened egg rougly 5cm long, 3cm wide and 2.5 cms thick and weighs 10-15 gms. They hang within the scrotum. During development the testes form inside the body cavity adjacent to the kidneys. As the foetus grows they move inferiorly and anteriorly towards the anterior abdominal wall. The gubernaculum testis is a cord of connective tissue and muscle fibers that extend from the inferior part of each testis to the posterior wall of a small, inferior pocket of the peritoneum. As growth proceeds the gubernacula do not elongate and the testes are held in position. During the seventh developmental month: growth continues at a rapid pace, circulating hormones stimulate contraction of the gubernaculum testis. Over this period the testes move through the abdominal musculature accompanied by small pockets of the peritoneal cavity. -

CHAPTER 6 Perineum and True Pelvis
193 CHAPTER 6 Perineum and True Pelvis THE PELVIC REGION OF THE BODY Posterior Trunk of Internal Iliac--Its Iliolumbar, Lateral Sacral, and Superior Gluteal Branches WALLS OF THE PELVIC CAVITY Anterior Trunk of Internal Iliac--Its Umbilical, Posterior, Anterolateral, and Anterior Walls Obturator, Inferior Gluteal, Internal Pudendal, Inferior Wall--the Pelvic Diaphragm Middle Rectal, and Sex-Dependent Branches Levator Ani Sex-dependent Branches of Anterior Trunk -- Coccygeus (Ischiococcygeus) Inferior Vesical Artery in Males and Uterine Puborectalis (Considered by Some Persons to be a Artery in Females Third Part of Levator Ani) Anastomotic Connections of the Internal Iliac Another Hole in the Pelvic Diaphragm--the Greater Artery Sciatic Foramen VEINS OF THE PELVIC CAVITY PERINEUM Urogenital Triangle VENTRAL RAMI WITHIN THE PELVIC Contents of the Urogenital Triangle CAVITY Perineal Membrane Obturator Nerve Perineal Muscles Superior to the Perineal Sacral Plexus Membrane--Sphincter urethrae (Both Sexes), Other Branches of Sacral Ventral Rami Deep Transverse Perineus (Males), Sphincter Nerves to the Pelvic Diaphragm Urethrovaginalis (Females), Compressor Pudendal Nerve (for Muscles of Perineum and Most Urethrae (Females) of Its Skin) Genital Structures Opposed to the Inferior Surface Pelvic Splanchnic Nerves (Parasympathetic of the Perineal Membrane -- Crura of Phallus, Preganglionic From S3 and S4) Bulb of Penis (Males), Bulb of Vestibule Coccygeal Plexus (Females) Muscles Associated with the Crura and PELVIC PORTION OF THE SYMPATHETIC -

Differential Diagnosis of Endometriosis by Ultrasound
diagnostics Review Differential Diagnosis of Endometriosis by Ultrasound: A Rising Challenge Marco Scioscia 1 , Bruna A. Virgilio 1, Antonio Simone Laganà 2,* , Tommaso Bernardini 1, Nicola Fattizzi 1, Manuela Neri 3,4 and Stefano Guerriero 3,4 1 Department of Obstetrics and Gynecology, Policlinico Hospital, 35031 Abano Terme, PD, Italy; [email protected] (M.S.); [email protected] (B.A.V.); [email protected] (T.B.); [email protected] (N.F.) 2 Department of Obstetrics and Gynecology, “Filippo Del Ponte” Hospital, University of Insubria, 21100 Varese, VA, Italy 3 Obstetrics and Gynecology, University of Cagliari, 09124 Cagliari, CA, Italy; [email protected] (M.N.); [email protected] (S.G.) 4 Department of Obstetrics and Gynecology, Azienda Ospedaliero Universitaria, Policlinico Universitario Duilio Casula, 09045 Monserrato, CA, Italy * Correspondence: [email protected] Received: 6 October 2020; Accepted: 15 October 2020; Published: 20 October 2020 Abstract: Ultrasound is an effective tool to detect and characterize endometriosis lesions. Variances in endometriosis lesions’ appearance and distorted anatomy secondary to adhesions and fibrosis present as major difficulties during the complete sonographic evaluation of pelvic endometriosis. Currently, differential diagnosis of endometriosis to distinguish it from other diseases represents the hardest challenge and affects subsequent treatment. Several gynecological and non-gynecological conditions can mimic deep-infiltrating endometriosis. For example, abdominopelvic endometriosis may present as atypical lesions by ultrasound. Here, we present an overview of benign and malignant diseases that may resemble endometriosis of the internal genitalia, bowels, bladder, ureter, peritoneum, retroperitoneum, as well as less common locations. An accurate diagnosis of endometriosis has significant clinical impact and is important for appropriate treatment. -

The Uterus and the Endometrium Common and Unusual Pathologies
The uterus and the endometrium Common and unusual pathologies Dr Anne Marie Coady Consultant Radiologist Head of Obstetric and Gynaecological Ultrasound HEY WACH Lecture outline Normal • Unusual Pathologies • Definitions – Asherman’s – Flexion – Osseous metaplasia – Version – Post ablation syndrome • Normal appearances – Uterus • Not covering congenital uterine – Cervix malformations • Dimensions Pathologies • Uterine – Adenomyosis – Fibroids • Endometrial – Polyps – Hyperplasia – Cancer To be avoided at all costs • Do not describe every uterus with two endometrial cavities as a bicornuate uterus • Do not use “malignancy cannot be excluded” as a blanket term to describe a mass that you cannot categorize • Do not use “ectopic cannot be excluded” just because you cannot determine the site of the pregnancy 2 Endometrial cavities Lecture outline • Definitions • Unusual Pathologies – Flexion – Asherman’s – Version – Osseous metaplasia • Normal appearances – Post ablation syndrome – Uterus – Cervix • Not covering congenital uterine • Dimensions malformations • Pathologies • Uterine – Adenomyosis – Fibroids • Endometrial – Polyps – Hyperplasia – Cancer Anteflexed Definitions 2 terms are described to the orientation of the uterus in the pelvis Flexion Version Flexion is the bending of the uterus on itself and the angle that the uterus makes in the mid sagittal plane with the cervix i.e. the angle between the isthmus: cervix/lower segment and the fundus Anteflexed < 180 degrees Retroflexed > 180 degrees Retroflexed Definitions 2 terms are described