Radiation, Multiple Dispersal and Parallelism in the Skinks, Chalcides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Southern Iraq Herpetofauna
Vol. 3 (1): 61-71, 2019 A Review of Southern Iraq Herpetofauna Nadir A. Salman Mazaya University College, Dhi Qar, Iraq *Corresponding author: [email protected] Abstract: The present review discussed the species diversity of herpetofauna in southern Iraq due to their scientific and national interests. The review includes a historical record for the herpetofaunal studies in Iraq since the earlier investigations of the 1920s and 1950s along with the more recent taxonomic trials in the following years. It appeared that, little is known about Iraqi herpetofauna, and no comprehensive checklist has been done for these species. So far, 96 species of reptiles and amphibians have been recorded from Iraq, but only a relatively small proportion of them occur in the southern marshes. The marshes act as key habitat for globally endangered species and as a potential for as yet unexplored amphibian and reptile diversity. Despite the lack of precise localities, the tree frog Hyla savignyi, the marsh frog Pelophylax ridibunda and the green toad Bufo viridis are found in the marshes. Common reptiles in the marshes include the Caspian terrapin (Clemmys caspia), the soft-shell turtle (Trionyx euphraticus), the Euphrates softshell turtle (Rafetus euphraticus), geckos of the genus Hemidactylus, two species of skinks (Trachylepis aurata and Mabuya vittata) and a variety of snakes of the genus Coluber, the spotted sand boa (Eryx jaculus), tessellated water snake (Natrix tessellata) and Gray's desert racer (Coluber ventromaculatus). More recently, a new record for the keeled gecko, Cyrtopodion scabrum and the saw-scaled viper (Echis carinatus sochureki) was reported. The IUCN Red List includes six terrestrial and six aquatic amphibian species. -

Trade-Offs Between Burrowing and Biting Force in Fossorial Scincid Lizards?
applyparastyle “fig//caption/p[1]” parastyle “FigCapt” Biological Journal of the Linnean Society, 2020, XX, 1–10. With 2 figures. Downloaded from https://academic.oup.com/biolinnean/advance-article-abstract/doi/10.1093/biolinnean/blaa031/5839769 by Museum National d'Histoire Naturelle user on 19 May 2020 Trade-offs between burrowing and biting force in fossorial scincid lizards? MARGOT LE GUILLOUX1, AURÉLIEN MIRALLES2, JOHN MEASEY3, BIEKE VANHOOYDONCK4, JAMES C. O’REILLY5, AURÉLIEN LOWIE6, and ANTHONY HERREL1,4,6,*, 1UMR 7179 C.N.R.S/M.N.H.N., Département Adaptations du Vivant, Bâtiment d’Anatomie Comparée, 55 rue Buffon, 75005, Paris, France 2Institut de Systématique, Evolution, Biodiversité, (UMR 7205 Muséum national d’Histoire naturelle, CNRS UPMC EPHE, Sorbonne Universités), CP30, 25 rue Cuvier 75005, Paris, France 3Centre for Invasion Biology, Department of Botany & Zoology, Stellenbosch University, Private Bag X1, 7602 Matieland, Stellenbosch, South Africa 4Deparment of Biology, University of Antwerp, Universiteitsplein 1, B2610 Antwerpen, Belgium 5Department of Biomedical Sciences, Ohio University, Cleveland Campus, SPS-334C, Cleveland, 45701 Ohio, USA 6Department of Biology, Evolutionary Morphology of Vertebrates, Ghent University, K.L. Ledeganckstraat 35, 9000 Ghent, Belgium Received 27 December 2019; revised 17 February 2020; accepted for publication 19 February 2020 Trade-offs are thought to be important in constraining evolutionary divergence as they may limit phenotypic diversification. The cranial system plays a vital role in many functions including defensive, territorial, predatory and feeding behaviours in addition to housing the brain and sensory systems. Consequently, the morphology of the cranial system is affected by a combination of selective pressures that may induce functional trade-offs. -

The Effects of Paleoclimate on the Distributions of Some North West African Lizards. Lee Ellis a Thesis Submitted in Partial
The effects of paleoclimate on the distributions of some North West African Lizards. Lee Ellis A thesis submitted in partial fulfilment of the requirements of Liverpool John Moores University for the degree of Master of Philosophy July 2018 1 Abstract As awareness grows regarding impacts of global climate change, so does concern over the effects these changes have on a species habitat and distribution. Climate change is thought to have a major effect on the distribution of species, with the potential to cause isolated/fragmented populations, which could lead to genetic divergence. In this study species distribution modelling was applied to species occurrence data on northwest African lizards from Morocco, with corresponding environmental data. The aim was to identify how intraspecific divergence might be related to historical climatic events. Species distribution models (SDMs) were used to quantify a species niche and define the constraining factors that affect that niche. SDMs predict areas of suitable habitat under different climatic scenarios that replicate prehistoric climates, and used to examine if there is evidence to suggest historical divergence or historical splits in distributions that correspond to current patterns of geographical divergence within species. MaxEnt was used to develop the SDMs and define the species niche and variable constraints. Previous studies have shown that the estimated divergence times of species discussed in this study range between 1–15 Ma. Environmental data dating back to these divergence times are unavailable or unreliable. Therefore, the Last Interglacial (LIG ~120,000 -140,000 years BP) and Last Glacial Maxima (LGM~ 21,000 years BP) datasets were used as a surrogate to earlier interglacial and glacial maximum climates, to analyse species distributions under earlier climatic scenarios which can then be inferred. -

Downloaded from Brill.Com10/06/2021 09:29:00AM Via Free Access 42 Luiselli Et Al
Contributions to Zoology, 74 (1/2) 41-49 (2005) Analysis of a herpetofaunal community from an altered marshy area in Sicily; with special remarks on habitat use (niche breadth and overlap), relative abundance of lizards and snakes, and the correlation between predator abundance and tail loss in lizards Luca Luiselli1, Francesco M. Angelici2, Massimiliano Di Vittorio3, Antonio Spinnato3, Edoardo Politano4 1 F.I.Z.V. (Ecology), via Olona 7, I-00198 Rome, Italy. E-mail: [email protected] 2 F.I.Z.V. (Mammalogy), via Cleonia 30, I-00152 Rome, Italy. 3 Via Jevolella 2, Termini Imprese (PA), Italy. 4 Centre of Environmental Studies ‘Demetra’, via Tomassoni 17, I-61032 Fano (PU), Italy Abstract relationships, thus rendering the examination of the relationships between predators and prey an extreme- A field survey was conducted in a highly degraded barren en- ly complicated task for the ecologist (e.g., see Con- vironment in Sicily in order to investigate herpetofaunal com- nell, 1975; May, 1976; Schoener, 1986). However, munity composition and structure, habitat use (niche breadth and there is considerable literature (both theoretical and overlap) and relative abundance of a snake predator and two spe- empirical) indicating that case studies of extremely cies of lizard prey. The site was chosen because it has a simple community structure and thus there is potentially less ecological simple communities, together with the use of appropri- complexity to cloud any patterns observed. We found an unexpect- ate minimal models, can help us to understand the edly high overlap in habitat use between the two closely related basis of complex patterns of ecological relationships lizards that might be explained either by a high competition for among species (Thom, 1975; Arditi and Ginzburg, space or through predator-mediated co-existence i.e. -

Exploring the Host Specificity and Diversity of Haemogregarines in the Canary Islands Beatriz Tomé1,2*, Ana Pereira1,2, Fátima Jorge3, Miguel A
Tomé et al. Parasites & Vectors (2018) 11:190 https://doi.org/10.1186/s13071-018-2760-5 RESEARCH Open Access Along for the ride or missing it altogether: exploring the host specificity and diversity of haemogregarines in the Canary Islands Beatriz Tomé1,2*, Ana Pereira1,2, Fátima Jorge3, Miguel A. Carretero1, D. James Harris1 and Ana Perera1 Abstract Background: Host-parasite relationships are expected to be strongly shaped by host specificity, a crucial factor in parasite adaptability and diversification. Because whole host communities have to be considered to assess host specificity, oceanic islands are ideal study systems given their simplified biotic assemblages. Previous studies on insular parasites suggest host range broadening during colonization. Here, we investigate the association between one parasite group (haemogregarines) and multiple sympatric hosts (of three lizard genera: Gallotia, Chalcides and Tarentola) in the Canary Islands. Given haemogregarine characteristics and insular conditions, we hypothesized low host specificity and/or occurrence of host-switching events. Methods: A total of 825 samples were collected from the three host taxa inhabiting the seven main islands of the Canarian Archipelago, including locations where the different lizards occurred in sympatry. Blood slides were screened to assess prevalence and parasitaemia, while parasite genetic diversity and phylogenetic relationships were inferred from 18S rRNA gene sequences. Results: Infection levels and diversity of haplotypes varied geographically and across host groups. Infections were found in all species of Gallotia across the seven islands, in Tarentola from Tenerife, La Gomera and La Palma, and in Chalcides from Tenerife, La Gomera and El Hierro. Gallotia lizards presented the highest parasite prevalence, parasitaemia and diversity (seven haplotypes), while the other two host groups (Chalcides and Tarentola) harbored one haplotype each, with low prevalence and parasitaemia levels, and very restricted geographical ranges. -

Khalladi-Bpp Anexes-Arabic.Pdf
Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 107 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 108 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 109 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 110 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 111 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 112 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 113 The IUCN Red List Categories and Criteria are intended to be an easily and widely understood system for classifying species at high risk of global extinction. The IUCN Red List is categorized in the following Categories: • Extinct (EX): A taxon is Extinct when there is no reasonable doubt that the last individual has died. A taxon is presumed Extinct when exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), throughout its historic range have failed to record an individual. Surveys should be over a time frame appropriate to the taxon’s life cycle and life form. Khalladi Windfarm and Power Line Projects 114 Biodiversity Protection Plan, July 2015 • Extinct in the Wild (EW): A taxon is Extinct in the Wild when it is known only to survive in cultivation, in captivity or as a naturalized population (or populations) well outside the past range. A taxon is presumed Extinct in the Wild when exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), throughout its historic range have failed to record an individual. -
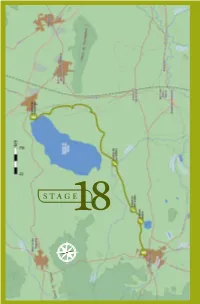
B I R D W a T C H I N G •
18 . F UENTE DE PIEDRA - CAMPILLOS STAGE18 E S N O 158 BIRDWATCHING • GR-249 Great Path of Malaga F UENTE DE PIEDRA - CAMPILLOS 18 . STAGE 18 Fuente de Piedra - Campillos L O C A T I O N he José Antonio Valverde Visitor´s Centre at the TReserva Natural de la Laguna de Fuente de Piedra is the starting point of Stage 18. Taking the direction south around the taken up mainly by olive trees and grain. eastern side of the salt water lagoon This type of environment will continue to you will be walking through farmland the end of this stage and it determines until the end of this stage in Campillos the species of birds which can be seen village. The 15, 7 km of Stage 18 will here. You will be crossing a stream and allow you to discover this wetland well then walking along the two lakes which known at national level in Spain, and will make your Stage 18 bird list fill cultivated farmland which creates a up with highly desirable species. The steppe-like environment. combination of wetland and steppe creates very valuable habitats with DESCRIPTION a rare composition of taxa unique at European level. ABOUT THE BIRDLIFE: Stage 18 begins at the northern tip HIGHLIGHTED SPECIES of the lagoon where you take direction Neither the length, diffi culty level or south through agricultural environment, elevation gain of this stage is particularly DID YOU KNOW? ecilio Garcia de la Leña, in Conversation 9th of “Historical Conversations of Malaga” published by Cristóbal Medina Conde (1726-1798) and entitled «About Animal Kingdom of Malaga and some Places in its Bishopric», Ccomments: «…In some lagoons, along sea shores and river banks some large and beautiful birds breed, called Flamingos and Phoenicopteros according to the ancients...», after a description of the bird´s anatomy he adds: «The Romans appreciated the bird greatly, especially its tongue which was served as an exquisite dish…». -

Setting Conservation Priorities for the Moroccan Herpetofauna: the Utility of Regional Red Listing
Oryx—The International Journal of Conservation Setting conservation priorities for the Moroccan herpetofauna: the utility of regional red listing J uan M. Pleguezuelos,JosE´ C. Brito,Soum´I A F ahd,Mo´ nica F eriche J osE´ A. Mateo,Gregorio M oreno-Rueda,Ricardo R eques and X avier S antos Appendix 1 Nomenclatural and taxonomical combinations scovazzi (Zangari et al., 2006) for amphibians. Chalcides for the Moroccan (Western Sahara included) amphibians lanzai (Caputo & Mellado, 1992), Leptotyphlops algeriensis and reptiles for which there are potential problems re- (Hahn & Wallach, 1998), Macroprotodon brevis (Carranza garding taxonomic combinations; species names are fol- et al., 2004) and Telescopus guidimakaensis (Bo¨hme et al., lowed by reference to publications that support these 1989) for reptiles are considered here as full species. Macro- combinations. The complete list of species is in Appendix 2. protodon abubakeri has been recently described for the Agama impalearis (Joger, 1991), Tarentola chazaliae region (Carranza et al., 2004) and Hemidactylus angulatus (Carranza et al., 2002), Chalcides boulengeri, Chalcides recently found within the limits of the study area (Carranza delislei, Chalcides sphenopsiformis (Carranza et al., 2008), & Arnold, 2006). Filtering was not applied to species of Timon tangitanus, Atlantolacerta andreanszkyi, Podarcis passive introduction into Morocco, such as Hemidactylus vaucheri, Scelarcis perspicillata (Arnold et al., 2007), turcicus and Hemidactylus angulatus. The three-toed skink Hyalosaurus koellikeri -

A New Miocene-Divergent Lineage of Old World Racer Snake from India
RESEARCH ARTICLE A New Miocene-Divergent Lineage of Old World Racer Snake from India Zeeshan A. Mirza1☯*, Raju Vyas2, Harshil Patel3☯, Jaydeep Maheta4, Rajesh V. Sanap1☯ 1 National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore 560065, India, 2 505, Krishnadeep Towers, Mission Road, Fatehgunj, Vadodra 390002, Gujarat, India, 3 Department of Biosciences, Veer Narmad South Gujarat University, Surat-395007, Gujarat, India, 4 Shree cultural foundation, Ahmedabad 380004, Gujarat, India ☯ These authors contributed equally to this work. * [email protected] Abstract A distinctive early Miocene-divergent lineage of Old world racer snakes is described as a new genus and species based on three specimens collected from the western Indian state of Gujarat. Wallaceophis gen. et. gujaratenesis sp. nov. is a members of a clade of old world racers. The monotypic genus represents a distinct lineage among old world racers is recovered as a sister taxa to Lytorhynchus based on ~3047bp of combined nuclear (cmos) and mitochondrial molecular data (cytb, ND4, 12s, 16s). The snake is distinct morphologi- cally in having a unique dorsal scale reduction formula not reported from any known colubrid snake genus. Uncorrected pairwise sequence divergence for nuclear gene cmos between OPEN ACCESS Wallaceophis gen. et. gujaratenesis sp. nov. other members of the clade containing old Citation: Mirza ZA, Vyas R, Patel H, Maheta J, world racers and whip snake is 21–36%. Sanap RV (2016) A New Miocene-Divergent Lineage of Old World Racer Snake from India. PLoS ONE 11 (3): e0148380. doi:10.1371/journal.pone.0148380 Introduction Editor: Ulrich Joger, State Natural History Museum, GERMANY Colubrid snakes are one of the most speciose among serpents with ~1806 species distributed across the world [1–5]. -

An Overview and Checklist of the Native and Alien Herpetofauna of the United Arab Emirates
Herpetological Conservation and Biology 5(3):529–536. Herpetological Conservation and Biology Symposium at the 6th World Congress of Herpetology. AN OVERVIEW AND CHECKLIST OF THE NATIVE AND ALIEN HERPETOFAUNA OF THE UNITED ARAB EMIRATES 1 1 2 PRITPAL S. SOORAE , MYYAS AL QUARQAZ , AND ANDREW S. GARDNER 1Environment Agency-ABU DHABI, P.O. Box 45553, Abu Dhabi, United Arab Emirates, e-mail: [email protected] 2Natural Science and Public Health, College of Arts and Sciences, Zayed University, P.O. Box 4783, Abu Dhabi, United Arab Emirates Abstract.—This paper provides an updated checklist of the United Arab Emirates (UAE) native and alien herpetofauna. The UAE, while largely a desert country with a hyper-arid climate, also has a range of more mesic habitats such as islands, mountains, and wadis. As such it has a diverse native herpetofauna of at least 72 species as follows: two amphibian species (Bufonidae), five marine turtle species (Cheloniidae [four] and Dermochelyidae [one]), 42 lizard species (Agamidae [six], Gekkonidae [19], Lacertidae [10], Scincidae [six], and Varanidae [one]), a single amphisbaenian, and 22 snake species (Leptotyphlopidae [one], Boidae [one], Colubridae [seven], Hydrophiidae [nine], and Viperidae [four]). Additionally, we recorded at least eight alien species, although only the Brahminy Blind Snake (Ramphotyplops braminus) appears to have become naturalized. We also list legislation and international conventions pertinent to the herpetofauna. Key Words.— amphibians; checklist; invasive; reptiles; United Arab Emirates INTRODUCTION (Arnold 1984, 1986; Balletto et al. 1985; Gasperetti 1988; Leviton et al. 1992; Gasperetti et al. 1993; Egan The United Arab Emirates (UAE) is a federation of 2007). -

Supp Table 1.Pdf
Supplementary Table S1. GC levels of rRNA from the five classes of vertebrates (a). Mammals Birds Reptiles Amphibians GC GC GC GC M10098 Homo sapiens 56.6 AF173637 Larus glaucoides 56.2 AY217906 Emoia jakati 55.9 EF376089 Hypsiboas raniceps 55.3 AJ311673 Equus caballus 56.2 AF173621 Anthracothorax nigricollis 56.1 AY217894 Acontias percivali 55.4 X04025 Xenopus laevis 53.8 AJ31167 Erinaceus europaeus 56.1 AF173619 Apus affinus 56.1 AY217900 Feylinia grandisquamis 54.7 EF364368 Atelopus flavescens 53.8 DQ222453 Bos taurus 56.1 AF173618 Gallirex porphyreolophus 56.1 AJ311672 Crocodylus niloticus 54.6 AF169014 Hyla chrysoscelis 53.3 X00686 Mus musculus 56.0 AF173617 Urocolius macrourus 56.1 AY217915 Sphenomorphus simus 54.4 AB239574 Cynops pyrrhogaster 53.1 AJ311674 Dasypus novemcinctus 56.0 AF173620 Bubo virginianus 56.0 AY217904 Gehyra mutilata 54.3 AF542043 Rana amurensis 52.8 AJ311679 Ornithorhynchus anatinus 55.9 AF173622 Chordeiles acutipennis 56.0 AY859624 Anolis carolinensis 54.2 AJ279506 Ranodon sibiricus 52.0 M11188 Rattus norvegicus 55.8 AF173636 Ciconia nigra 56.0 AY217939 Eumeces inexpectatus 54.2 DQ235090 Cricetulus griseus 55.7 AF173630 Columba livia 56.0 AY21793 Scelotes kasneri 54.2 AJ311676 Monodelphis domestica 55.7 AF173625 Coracias caudata 56.0 AY217924 Scincus scincus 54.2 AJ311677 Didelphis virginiana 55.7 AF173629 Melopsittacus undulatus 56.0 AY217911 Mabuya hoeschi 54.2 AJ311678 Vombatus ursinus 55.6 AF173633 Neophron percnopterus 56.0 AY217909 Eugongylus rufescens 54.2 X06778 Oryctolagus cuniculus 55.3 AF173613 Ortalis -

Declive De La Población Más Noroccidental De Chalcides Bedriagai
Bol. Asoc. Herpetol. Esp. (2019) 30(1) 79 Declive de la población más noroccidental de Chalcides bedriagai Pedro Galán Grupo de Investigación en Bioloxía Evolutiva (GIBE). Departamento de Bioloxía. Facultade de Ciencias. Universidade da Coruña. Cam- pus da Zapateira, s/n. 15071 A Coruña. España. C.e.: [email protected] Fecha de aceptación: 10 de junio de 2019. Key words: skinks, lizards, extinction risk, Galicia, threatened populations, wild boar depredation. Chalcides bedriagai es un reptil endémi- Entre los meses de abril y agosto de 2016 co de la península ibérica cuya distribución y 2018 y entre abril y mayo de 2019, se pros- es relativamente amplia dentro de ésta, pectaron las zonas interiores arenosas vegeta- aunque se encuentra ausente de gran parte das de las playas de Lariño y el extremo norte de su tercio norte (Pollo, 2004; Salvador, 2014). de la de Area Maior (Ancoradoiro), espacios En Galicia, donde está el extremo noroc- que constituyen el núcleo principal de esta cidental de su distribución geográfica, las población de eslizón ibérico. Se muestrearon poblaciones son escasas y se encuentran los ecotonos entre las dunas secundarias y los dispersas en su mitad sur (Sociedade Galega de matorrales y herbazales de trasduna. Debi- Historia Natural, 2019), localizándose la más do a que esta especie raramente es observada extrema noroccidental en la costa meridio- activa, la búsqueda se realizó levantando las nal de A Coruña, en las playas de Louro y numerosas piedras que se encuentran sobre Lariño (Galán & Fernández-Arias, 1993; Galán, el suelo arenoso en estas zonas, restos de an- 2003; Serantes & Galán, 2007).