Growth and Survival of Satellite Glial Cells in Different Culture Supplementations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
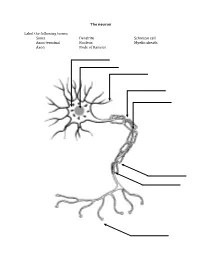
The Neuron Label the Following Terms: Soma Axon Terminal Axon Dendrite
The neuron Label the following terms: Soma Dendrite Schwaan cell Axon terminal Nucleus Myelin sheath Axon Node of Ranvier Neuron Vocabulary You must know the definitions of these terms 1. Synaptic Cleft 2. Neuron 3. Impulse 4. Sensory Neuron 5. Motor Neuron 6. Interneuron 7. Body (Soma) 8. Dendrite 9. Axon 10. Action Potential 11. Myelin Sheath (Myelin) 12. Afferent Neuron 13. Threshold 14. Neurotransmitter 15. Efferent Neurons 16. Axon Terminal 17. Stimulus 18. Refractory Period 19. Schwann 20. Nodes of Ranvier 21. Acetylcholine STEPS IN THE ACTION POTENTIAL 1. The presynaptic neuron sends neurotransmitters to postsynaptic neuron. 2. Neurotransmitters bind to receptors on the postsynaptic cell. - This action will either excite or inhibit the postsynaptic cell. - The soma becomes more positive. 3. The positive charge reaches the axon hillock. - Once the threshold of excitation is reached the neuron will fire an action potential. 4. Na+ channels open and Na+ is forced into the cell by the concentration gradient and the electrical gradient. - The neuron begins to depolarize. 5. The K+ channels open and K+ is forced out of the cell by the concentration gradient and the electrical gradient. - The neuron is depolarized. 6. The Na+ channels close at the peak of the action potential. - The neuron starts to repolarize. 7. The K+ channels close, but they close slowly and K+ leaks out. 8. The terminal buttons release neurotransmitter to the postsynaptic neuron. 9. The resting potential is overshot and the neuron falls to a -90mV (hyperpolarizes). - The neuron continues to repolarize. 10. The neuron returns to resting potential. The Synapse Label the following terms: Pre-synaptic membrane Neurotransmitters Post-synaptic membrane Synaptic cleft Vesicle Post-synaptic receptors . -

Crayfish Escape Behavior and Central Synapses
Crayfish Escape Behavior and Central Synapses. III. Electrical Junctions and Dendrite Spikes in Fast Flexor Motoneurons ROBERT S. ZUCKER Department of Biological Sciences and Program in the Neurological Sciences, Stanford University, Stanford, California 94305 THE LATERAL giant fiber is the decision fiber and fast flexor motoneurons are located in for a behavioral response in crayfish in- the dorsal neuropil of each abdominal gan- volving rapid tail flexion in response to glion. Dye injections and anatomical recon- abdominal tactile stimulation (27). Each structions of ‘identified motoneurons reveal lateral giant impulse is followed by a rapid that only those motoneurons which have tail flexion or tail flip, and when the giant dentritic branches in contact with a giant fiber does not fire in response to such stim- fiber are activated by that giant fiber (12, uli, the movement does not occur. 21). All of tl ie fast flexor motoneurons are The afferent limb of the reflex exciting excited by the ipsilateral giant; all but F4, the lateral giant has been described (28), F5 and F7 are also excited by the contra- and the habituation of the behavior has latkral giant (21 a). been explained in terms of the properties The excitation of these motoneurons, of this part of the neural circuit (29). seen as depolarizing potentials in their The properties of the neuromuscular somata, does not always reach threshold for junctions between the fast flexor motoneu- generating a spike which propagates out the rons and the phasic flexor muscles have also axon to the periphery (14). Indeed, only t,he been described (13). -

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Oligodendrocytes in Development, Myelin Generation and Beyond
cells Review Oligodendrocytes in Development, Myelin Generation and Beyond Sarah Kuhn y, Laura Gritti y, Daniel Crooks and Yvonne Dombrowski * Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK; [email protected] (S.K.); [email protected] (L.G.); [email protected] (D.C.) * Correspondence: [email protected]; Tel.: +0044-28-9097-6127 These authors contributed equally. y Received: 15 October 2019; Accepted: 7 November 2019; Published: 12 November 2019 Abstract: Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that are generated from oligodendrocyte progenitor cells (OPC). OPC are distributed throughout the CNS and represent a pool of migratory and proliferative adult progenitor cells that can differentiate into oligodendrocytes. The central function of oligodendrocytes is to generate myelin, which is an extended membrane from the cell that wraps tightly around axons. Due to this energy consuming process and the associated high metabolic turnover oligodendrocytes are vulnerable to cytotoxic and excitotoxic factors. Oligodendrocyte pathology is therefore evident in a range of disorders including multiple sclerosis, schizophrenia and Alzheimer’s disease. Deceased oligodendrocytes can be replenished from the adult OPC pool and lost myelin can be regenerated during remyelination, which can prevent axonal degeneration and can restore function. Cell population studies have recently identified novel immunomodulatory functions of oligodendrocytes, the implications of which, e.g., for diseases with primary oligodendrocyte pathology, are not yet clear. Here, we review the journey of oligodendrocytes from the embryonic stage to their role in homeostasis and their fate in disease. We will also discuss the most common models used to study oligodendrocytes and describe newly discovered functions of oligodendrocytes. -

Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex Ralf S. Schmid*, Barbara McGrath*, Bridget E. Berechid†, Becky Boyles*, Mark Marchionni‡, Nenad Sˇ estan†, and Eva S. Anton*§ *University of North Carolina Neuroscience Center and Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, NC 27599; †Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510; and ‡CeNes Pharamceuticals, Inc., Norwood, MA 02062 Communicated by Pasko Rakic, Yale University School of Medicine, New Haven, CT, January 27, 2003 (received for review December 12, 2002) Radial glial cells and astrocytes function to support the construction mine whether NRG-1-mediated signaling is involved in radial and maintenance, respectively, of the cerebral cortex. However, the glial cell development and differentiation in the cerebral cortex. mechanisms that determine how radial glial cells are established, We show that NRG-1 signaling, involving erbB2, may act in maintained, and transformed into astrocytes in the cerebral cortex are concert with Notch signaling to exert a critical influence in the not well understood. Here, we show that neuregulin-1 (NRG-1) exerts establishment, maintenance, and appropriate transformation of a critical role in the establishment of radial glial cells. Radial glial cell radial glial cells in cerebral cortex. generation is significantly impaired in NRG mutants, and this defect can be rescued by exogenous NRG-1. Down-regulation of expression Materials and Methods and activity of erbB2, a member of the NRG-1 receptor complex, leads Clonal Analysis to Study NRG’s Role in the Initial Establishment of to the transformation of radial glial cells into astrocytes. -

Build a Neuron
Build a Neuron Objectives: 1. To understand what a neuron is and what it does 2. To understand the anatomy of a neuron in relation to function This activity is great for ALL ages-even college students!! Materials: pipe cleaners (2 full size, 1 cut into 3 for each student) pony beads (6/student Introduction: Little kids: ask them where their brain is (I point to my head and torso areas till they shake their head yes) Talk about legos being the building blocks for a tower and relate that to neurons being the building blocks for your brain and that neurons send messages to other parts of your brain and to and from all your body parts. Give examples: touch from body to brain, movement from brain to body. Neurons are the building blocks of the brain that send and receive messages. Neurons come in all different shapes. Experiment: 1. First build soma by twisting a pipe cleaner into a circle 2. Then put a 2nd pipe cleaner through the circle and bend it over and twist the two strands together to make it look like a lollipop (axon) 3. take 3 shorter pipe cleaners attach to cell body to make dendrites 4. add 6 beads on the axon making sure there is space between beads for the electricity to “jump” between them to send the signal super fast. (myelin sheath) 5. Twist the end of the axon to make it look like 2 feet for the axon terminal. 6. Make a brain by having all of the neurons “talk” to each other (have each student hold their neuron because they’ll just throw them on a table for you to do it.) messages come in through the dendrites and if its a strong enough electrical change, then the cell body sends the Build a Neuron message down it’s axon where a neurotransmitter is released. -

The Interplay Between Neurons and Glia in Synapse Development And
Available online at www.sciencedirect.com ScienceDirect The interplay between neurons and glia in synapse development and plasticity Jeff A Stogsdill and Cagla Eroglu In the brain, the formation of complex neuronal networks and regulate distinct aspects of synaptic development and amenable to experience-dependent remodeling is complicated circuit connectivity. by the diversity of neurons and synapse types. The establishment of a functional brain depends not only on The intricate communication between neurons and glia neurons, but also non-neuronal glial cells. Glia are in and their cooperative roles in synapse formation are now continuous bi-directional communication with neurons to direct coming to light due in large part to advances in genetic the formation and refinement of synaptic connectivity. This and imaging tools. This article will examine the progress article reviews important findings, which uncovered cellular made in our understanding of the role of mammalian and molecular aspects of the neuron–glia cross-talk that perisynaptic glia (astrocytes and microglia) in synapse govern the formation and remodeling of synapses and circuits. development, maturation, and plasticity since the previ- In vivo evidence demonstrating the critical interplay between ous Current Opinion article [1]. An integration of past and neurons and glia will be the major focus. Additional attention new findings of glial control of synapse development and will be given to how aberrant communication between neurons plasticity is tabulated in Box 1. and glia may contribute to neural pathologies. Address Glia control the formation of synaptic circuits Department of Cell Biology, Duke University Medical Center, Durham, In the CNS, glial cells are in tight association with NC 27710, USA synapses in all brain regions [2]. -

Neurons – Is a Basic Cell of the Nervous System. • Neurons Carry Nerve Messages, Or Impulses, from One Part of the Body to Another
Nervous System Nerves and Nerve Cells: Neurons – is a basic cell of the nervous system. • Neurons carry nerve messages, or impulses, from one part of the body to another. Structure of a Nerve Cell: A neuron has three basic parts: 1. Body – controls the cell’s growth 2. Axon – is a long thin fiber that carries impulses away from the cell body Myelin – is a fatty material that insulates the axon and increases the speed at which an impulse travels 3. Dendrites – are short, branching fibers that carry nerve impulses toward the cell body. • A nerve impulse begins when the dendrites are stimulated. The impulse travels along the dendrites to the cell body, and then away from the cell body on the axon. • The impulse must cross a synapse to a muscle or another neuron. Synapse – is the space between an axon and the structure with which the neuron communicates. Types of Nerve Cells: Sensory Neurons – pick up information about your external and internal environment from your sense organs and your body Motor Neurons – sends impulses to your muscles and glands, causing them to react Interneurons – are located only in the brain and spinal cord, pass impulses from one neuron to another The Central Nervous System: • The nervous system consists of two parts. Your brain and spinal cord make up one part, which is called the central nervous system. 1 • The peripheral nervous system, which is the other part, is made up of all the nerves that connect the brain and spinal cord to other parts of the body. The Brain: Brain ± a moist, spongy organ weighing about three pounds is made up of billions of neurons that control almost everything you do and experience. -

Quiescent Satellite Glial Cells of the Adult Trigeminal Ganglion
Cent. Eur. J. Med. • 9(3) • 2014 • 500-504 DOI: 10.2478/s11536-013-0285-z Central European Journal of Medicine Quiescent satellite glial cells of the adult trigeminal ganglion Research Article Mugurel C. Rusu*1,2,3, Valentina M. Mănoiu4, Nicolae Mirancea3, Gheorghe Nini5 1 „Carol Davila” University of Medicine and Pharmacy, 050511 Bucharest, Romania. 2 MEDCENTER - Center of Excellence in Laboratory Medicine and Pathology 013594 Bucharest, Romania 3 Institute of Biology of Bucharest – The Romanian Academy, , 060031 Bucharest, Romania 4 Faculty of Geography, University of Bucharest, 050107 Bucharest, Romania 5 Faculty of Medicine, Pharmacy and Dental Medicine, “Vasile Goldiş” Western University, 310045 Arad, Romania Received 18 August 2013; Accepted 27 November 2013 Abstract: Sensory ganglia comprise functional units built up by neurons and satellite glial cells (SGCs). In animal species there was proven the presence of neuronoglial progenitor cells in adult samples. Such neural crest-derived progenitors were found in immunohistochemistry (IHC). These fi ndings were not previously documented in transmission electron microscopy (TEM). It was thus aimed to assess in TEM if cells of the human adult trigeminal ganglion indeed have ultrastructural features to qualify for a progenitor, or quiescent phenotype. Trigeminal ganglia were obtained from fi fteen adult donor cadavers. In TEM, cells with heterochromatic nuclei, a pancytoplasmic content of free ribosomes, few perinuclear mitochondria, poor developed endoplasmic reticulum, lack of Golgi complexes and membrane traffi cking specializations, were found included in the neuronal envelopes built-up by SGCs. The ultrastructural pattern was strongly suggestive for these cells being quiescent progenitors. However, further experiments should correlate the morphologic and immune phenotypes of such cells. -

The Myelin-Forming Cells of the Nervous System (Oligodendrocytes and Schwann Cells)
The Myelin-Forming Cells of the Nervous System (oligodendrocytes and Schwann cells) Oligodendrocyte Schwann Cell Oligodendrocyte function Saltatory (jumping) nerve conduction Oligodendroglia PMD PMD Saltatory (jumping) nerve conduction Investigating the Myelinogenic Potential of Individual Oligodendrocytes In Vivo Sparse Labeling of Oligodendrocytes CNPase-GFP Variegated expression under the MBP-enhancer Cerebral Cortex Corpus Callosum Cerebral Cortex Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Ant Commissure Corpus Callosum Cerebral Cortex Caudate Putamen Piriform Cortex Corpus Callosum Ant Commissure Characterization of Oligodendrocyte Morphology Cerebral Cortex Corpus Callosum Caudate Putamen Cerebellum Brain Stem Spinal Cord Oligodendrocytes in disease: Cerebral Palsy ! CP major cause of chronic neurological morbidity and mortality in children ! CP incidence now about 3/1000 live births compared to 1/1000 in 1980 when we started intervening for ELBW ! Of all ELBW {gestation 6 mo, Wt. 0.5kg} , 10-15% develop CP ! Prematurely born children prone to white matter injury {WMI}, principle reason for the increase in incidence of CP ! ! 12 Cerebral Palsy Spectrum of white matter injury ! ! Macro Cystic Micro Cystic Gliotic Khwaja and Volpe 2009 13 Rationale for Repair/Remyelination in Multiple Sclerosis Oligodendrocyte specification oligodendrocytes specified from the pMN after MNs - a ventral source of oligodendrocytes -

Canine Dorsal Root Ganglia Satellite Glial Cells Represent an Exceptional Cell Population with Astrocytic and Oligodendrocytic P
www.nature.com/scientificreports OPEN Canine dorsal root ganglia satellite glial cells represent an exceptional cell population with astrocytic and Received: 17 August 2017 Accepted: 6 October 2017 oligodendrocytic properties Published: xx xx xxxx W. Tongtako1,2, A. Lehmbecker1, Y. Wang1,2, K. Hahn1,2, W. Baumgärtner1,2 & I. Gerhauser 1 Dogs can be used as a translational animal model to close the gap between basic discoveries in rodents and clinical trials in humans. The present study compared the species-specifc properties of satellite glial cells (SGCs) of canine and murine dorsal root ganglia (DRG) in situ and in vitro using light microscopy, electron microscopy, and immunostainings. The in situ expression of CNPase, GFAP, and glutamine synthetase (GS) has also been investigated in simian SGCs. In situ, most canine SGCs (>80%) expressed the neural progenitor cell markers nestin and Sox2. CNPase and GFAP were found in most canine and simian but not murine SGCs. GS was detected in 94% of simian and 71% of murine SGCs, whereas only 44% of canine SGCs expressed GS. In vitro, most canine (>84%) and murine (>96%) SGCs expressed CNPase, whereas GFAP expression was diferentially afected by culture conditions and varied between 10% and 40%. However, GFAP expression was induced by bone morphogenetic protein 4 in SGCs of both species. Interestingly, canine SGCs also stimulated neurite formation of DRG neurons. These fndings indicate that SGCs represent an exceptional, intermediate glial cell population with phenotypical characteristics of oligodendrocytes and astrocytes and might possess intrinsic regenerative capabilities in vivo. Since the discovery of glial cells over a century ago, substantial progress has been made in understanding the origin, development, and function of the diferent types of glial cells in the central nervous system (CNS) and peripheral nervous system (PNS)1. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria.