Morphological and Structural Diversities of Indigenous Endomycorrhiza Communities Associated to Maize [Zea Mays (L.)] in Norther
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cameroon : Adamawa, East and North Rgeions
CAMEROON : ADAMAWA, EAST AND NORTH RGEIONS 11° E 12° E 13° E 14° E N 1125° E 16° E Hossere Gaval Mayo Kewe Palpal Dew atan Hossere Mayo Kelvoun Hossere HDossere OuIro M aArday MARE Go mbe Trabahohoy Mayo Bokwa Melendem Vinjegel Kelvoun Pandoual Ourlang Mayo Palia Dam assay Birdif Hossere Hosere Hossere Madama CHARI-BAGUIRMI Mbirdif Zaga Taldam Mubi Hosere Ndoudjem Hossere Mordoy Madama Matalao Hosere Gordom BORNO Matalao Goboum Mou Mayo Mou Baday Korehel Hossere Tongom Ndujem Hossere Seleguere Paha Goboum Hossere Mokoy Diam Ibbi Moukoy Melem lem Doubouvoum Mayo Alouki Mayo Palia Loum as Marma MAYO KANI Mayo Nelma Mayo Zevene Njefi Nelma Dja-Lingo Birdi Harma Mayo Djifi Hosere Galao Hossere Birdi Beli Bili Mandama Galao Bokong Babarkin Deba Madama DabaGalaou Hossere Goudak Hosere Geling Dirtehe Biri Massabey Geling Hosere Hossere Banam Mokorvong Gueleng Goudak Far-North Makirve Dirtcha Hwoli Ts adaksok Gueling Boko Bourwoy Tawan Tawan N 1 Talak Matafal Kouodja Mouga Goudjougoudjou MasabayMassabay Boko Irguilang Bedeve Gimoulounga Bili Douroum Irngileng Mayo Kapta Hakirvia Mougoulounga Hosere Talak Komboum Sobre Bourhoy Mayo Malwey Matafat Hossere Hwoli Hossere Woli Barkao Gande Watchama Guimoulounga Vinde Yola Bourwoy Mokorvong Kapta Hosere Mouga Mouena Mayo Oulo Hossere Bangay Dirbass Dirbas Kousm adouma Malwei Boulou Gandarma Boutouza Mouna Goungourga Mayo Douroum Ouro Saday Djouvoure MAYO DANAY Dum o Bougouma Bangai Houloum Mayo Gottokoun Galbanki Houmbal Moda Goude Tarnbaga Madara Mayo Bozki Bokzi Bangei Holoum Pri TiraHosere Tira -

Emergency Appeal Operation Update Cameroon: Floods
Emergency appeal operation update Cameroon: Floods Emergency appeal n° MDRCM014 GLIDE n° FL-2012-000157-CMR Operation update n°1 19 November, 2012 Period covered by this Ops Update: 6 September to 31 October, 2012. Appeal target (current): CHF 1,637,316. Appeal coverage: 18%; not including DREF allocation and yet-to-be-confirmed pledges. <Click here to go directly to the updated donor response report; here for interim financial here to link to contact details > Appeal history: This Emergency Appeal was initially launched on 28 September, 2012 for CHF 1,637,314 for 12 months to assist about 25,000 beneficiaries. Disaster Relief Emergency Fund (DREF): CHF 299,707 was initially allocated from the Federation’s DREF to Cameroon Red Cross volunteers constructing emergency latrines in support the national society to respond. Northern Cameroon with assistance from members of the floods affected community / Photo by Cameroon Red Cross Summary: Beginning the second half of August 2012, widespread heavy rain in Cameroon caused severe flooding, especially in North, and Far North regions. IFRC helped the Cameroon Red Cross to obtain DREF funds in order to assist the affected populations in the North and Far North Regions. Considering the magnitude of the situation, this DREF was transformed into an emergency appeal. Funds raised through this emergency appeal, in addition to those of the DREF, have so far enabled the Cameroon Red Cross to build 170 emergency shelters for internally displaced persons (IDPs), build 26 emergency latrines, distribute non-food items to 921 families, treat 198 wells and provide first aid to flood victims in the two regions. -

Cameroon: Cholera 21 April, 2010
DREF operation n° DRMDRCM007 GLIDE n° EP-2009-000021-CM Cameroon: Cholera 21 April, 2010 The International Federation of Red Cross and Red Crescent (IFRC) Disaster Relief Emergency Fund (DREF) is a source of un-earmarked money created by the Federation in 1985 to ensure that immediate financial support is available for Red Cross Red Crescent response to emergencies. The DREF is a vital part of the International Federation’s disaster response system and increases the ability of National Societies to respond to disasters. Summary: CHF 203,419 was allocated from the IFRC’s Disaster Relief Emergency Fund (DREF) on 26 October, 2009 to support the Cameroon Red Cross national society in delivering assistance to some 800,000 beneficiaries. Twenty health districts were hit by a cholera outbreak in the North and Far North regions of Cameroon in October 2009. The funds allocated from DREF enabled the Cameroon Red Cross National Society to train 495 volunteers who in turn conducted health education in the various neighbourhoods affected. In addition, the identified cholera cases were referred to the nearest health centres. The trained volunteers also distributed sanitation materials which were used to disinfect latrines, treat water Cameroon Red Cross volunteers covered water wells with metal roofing sheets to prevent them from being contaminated/ Viviane points, clean gutters and organise Nzeusseu/IFRC cleanliness days in the market places of the affected localities. In addition, buckets with covers and taps were distributed to restaurants in the affected localities for clients to wash their hands. Red Cross volunteers also distributed metal roofing sheets to cover latrines and water wells, and chemicals for disinfecting water. -

Cameroon Humanitarian Situation Report
Cameroon Humanitarian Situation Report ©UNICEF Cameroon/2019 SITUATION IN NUMBERS Highlights August 2019 2,300,000 • More than 118,000 people have benefited from UNICEF’s # of children in need of humanitarian assistance humanitarian assistance in the North-West and South-West 4,300,000 regions since January including 15,800 in August. # of people in need (Cameroon Humanitarian Needs Overview 2019) • The Rapid Response Mechanism (RRM) strategy, Displacement established in the South-West region in June, was extended 530,000 into the North-West region in which 1,640 people received # of Internally Displaced Persons (IDPs) in the North- WASH kits and Long-Lasting Insecticidal Nets (LLINs) in West and South-West regions (OCHA Displacement Monitoring, July 2019) August. 372,854 # of IDPs and Returnees in the Far-North region • In August, 265,694 children in the Far-North region were (IOM Displacement Tracking Matrix 18, April 2019) vaccinated against poliomyelitis during the final round of 105,923 the vaccination campaign launched following the polio # of Nigerian Refugees in rural areas (UNHCR Fact Sheet, July 2019) outbreak in May. UNICEF Appeal 2019 • During the month of August, 3,087 children received US$ 39.3 million psychosocial support in the Far-North region. UNICEF’s Response with Partners Total funding Funds requirement received Sector Total UNICEF Total available 20% $ 4.5M Target Results* Target Results* Carry-over WASH: People provided with 374,758 33,152 75,000 20,181 $ 3.2 M access to appropriate sanitation 2019 funding Education: Number of boys and requirement: girls (3 to 17 years) affected by 363,300 2,415 217,980 0 $39.3 M crisis receiving learning materials Nutrition**: Number of children Funding gap aged 6-59 months with SAM 60,255 39,727 65,064 40,626 $ 31.6M admitted for treatment Child Protection: Children reached with psychosocial support 563,265 160,423 289,789 87,110 through child friendly/safe spaces C4D: Persons reached with key life- saving & behaviour change 385,000 431,034 messages *Total results are cumulative. -

Proceedingsnord of the GENERAL CONFERENCE of LOCAL COUNCILS
REPUBLIC OF CAMEROON REPUBLIQUE DU CAMEROUN Peace - Work - Fatherland Paix - Travail - Patrie ------------------------- ------------------------- MINISTRY OF DECENTRALIZATION MINISTERE DE LA DECENTRALISATION AND LOCAL DEVELOPMENT ET DU DEVELOPPEMENT LOCAL Extrême PROCEEDINGSNord OF THE GENERAL CONFERENCE OF LOCAL COUNCILS Nord Theme: Deepening Decentralization: A New Face for Local Councils in Cameroon Adamaoua Nord-Ouest Yaounde Conference Centre, 6 and 7 February 2019 Sud- Ouest Ouest Centre Littoral Est Sud Published in July 2019 For any information on the General Conference on Local Councils - 2019 edition - or to obtain copies of this publication, please contact: Ministry of Decentralization and Local Development (MINDDEVEL) Website: www.minddevel.gov.cm Facebook: Ministère-de-la-Décentralisation-et-du-Développement-Local Twitter: @minddevelcamer.1 Reviewed by: MINDDEVEL/PRADEC-GIZ These proceedings have been published with the assistance of the German Federal Ministry for Economic Cooperation and Development (BMZ) through the Deutsche Gesellschaft für internationale Zusammenarbeit (GIZ) GmbH in the framework of the Support programme for municipal development (PROMUD). GIZ does not necessarily share the opinions expressed in this publication. The Ministry of Decentralisation and Local Development (MINDDEVEL) is fully responsible for this content. Contents Contents Foreword ..............................................................................................................................................................................5 -

NZB Newsletter
Entomology Laboratory BORDER HEALTH NEWSLETTER - FEBRUARY 2012 WELCOME! SAMPLES Hi everyone. It’s now the end of summer - did During February, 653 samples were collected you blink and miss it? The mozzies are still out by staff from 12 District Health Boards, with there doing their best in spite of the 218 positive. Sampling numbers were almost temperatures and the recent rainfall will be identical to last month (652) but with more helping them boost their numbers. positives this month and well down on this time last year. The specimens received were: Please remember if you have new staff or people leaving to let us know Species Adults Larvae ([email protected]) so we can NZ Mozzies update our distribution list and ensure the Aedes antipodeus 4 1 newsletter is getting sent to the right people. Ae. australis 0 46 Ae. notoscriptus 1497 2793 INCURSIONS/INTERCEPTIONS Coquillettidia iracunda 21 0 There were no interception callouts during Culex pervigilans 183 2020 March. Cx. quinquefasciatus 121 2336 Opifex fuscus 2 112 WEBSITE The SMS NZB website has had a lot of traffic as Exotics 0 0 a result of the December interception of Aedes aegypti and Aedes polynesiensis. Species TOTAL MOSQUITOES 1828 7308 profiles in particular are of interest, we think this is a great indication that the public are very interested in keeping New Zealand free of MOSQUITO-BORNE DISEASES exotic mosquitoes. The species profiles provide current information and an ideal source that the INVASIVE MOSQUITOES - UK public can be directed to for more detail on Source: Mirror [edited], Thu 9 Feb 2012 reported mosquito species. -

Consideration of Reports Submitted by States Parties Under Article 19 of the Convention
United Nations CAT/C/CMR/4 Convention against Torture Distr.: General 5 August 2009 and Other Cruel, Inhuman English or Degrading Treatment Original: French or Punishment Committee against Torture Consideration of reports submitted by States parties under article 19 of the Convention Fourth periodic report of States parties due in 2000 Cameroon* ** [27 November 2008] * For the third periodic report, see CAT/C/34/Add.17; for its consideration by the Committee on 18, 19 and 20 November 2003, see CAT/C/SR.585, 588 and 590. ** The annexes to the present report may be consulted in the files of the secretariat. GE.09-44036 (E) 141209 231209 CAT/C/CMR/4 Contents Abbreviations and acronyms Chapter Paragraphs Page Introduction............................................................................................................. 1–2 4 I. New information on the general framework for implementation of the Convention in domestic law.................................................................................... 3–20 4 A. Normative measures ....................................................................................... 4–9 4 B. Institutional measures..................................................................................... 10–20 6 II. Responses to the Committee’s recommendations................................................... 21–147 8 A. Response of the State of Cameroon to the recommendations in paragraph 8 of the Committee’s concluding observations.............................. 21–81 8 B. Response of the State of Cameroon -

N.Pmruixior Uurrrm DIRECTION DES RESSOURCES RESOURCES HUMAINES
REPUBLIQUE DU CAMEROUN Rf,PUBLTC OF CAMEROON Paix-Travail-Patrie Peace.Work-Fatherlând MINISTERE DE L'EDUCATION DE MINISTRY OF BASIC EDUCATION BASE GENERAL SECRETARIAT SECRETARIAT Gf,NERAL n.pmruixior uurrrm DIRECTION DES RESSOURCES RESOURCES HUMAINES TROISIEME PROGRAMME DE CONTRACTUALISATION DES INSTITUTEURS AU MINISTERE DE L'EDUCATION DE BASE DEUXIEME OPERATION AU TITRE DE L'EXERCICE 2O2O usrE DEs EcoLEs NECESSTTEUSES (JOB POSTING ) REGION DU NORD N" REGION DEPARTEMENT ARRONDISSEMENT NOM DE L'ECOLE 1 NORD BENOUE BASCHEO ECOLE PUBLIQUE DE BASCHEO 2 NORD BEN OU E BASCHEO EP BANTAYE-BASCH EO 3 NORD BE NOU E BASCHEO EP BARGOUMA 4 NORD BENOUE BASCHEO EP BOMBOL 5 NORD BENOU E BASCHEO EP DARAM 6 NORD BE NOU E BASCHEO EP DARPATA 7 NORD BE NOU E BASCHEO EP DJALINGO.BELEL 8 NORD BEN OU E BASCHEO EP DJAOURO-MOUSSA 9 NORD BEN OU E BASCHEO EP DJARENGOL 10 NORD BEN OU E BASCHEO EP DORBA 11 NORD B ENOU E BASCHEO EP FOULBERE 72 NORD BENOUE BASCHEO EP GASSIRE -15 NORD B ENOU E BASCHEO EP HAMAKOUSSOU 14 NORD B ENOU E BASCHEO EP HAMAYEL 15 NORD BENO U E BASCHEO EP HARKOU NORD BENOUE BASCHEO EP KATAKO 17 NORD 8 ENOU E BASCHEO EP KATCHATCHIA 18 NORD BENOUE BASCHEO EP KERZING 19 NORD BENOUE EASCHEO EP KOBOSSI 20 NORD B ENOU E BASCHEO EP LARIA 2t NORD BENOUE BASCHEO EP MALKOUROU NORD BENOU E BASCHEO EP MAPOUTKI 23 NORD BENOUE BASCHEO EP MAYO-OULO-FALI 24 NORD BENOUE BASCHEO EP MBABI 25 NORD BENOUE BASCHEO EP MBOULMI 26 NORD BENOUE BASCHEO EP NARO-KOUBADJE 27 NORD 8 ENOU E BASCHEO EP NASSARAO 28 NORD BENOUE BASCHEO EP NGOUROU-DABA 29 NORD BENOUE BASCHEO EP -

Rapport De Presentation Des Resultats Definitifs Resume
RAPPORT DE PRESENTATION DES RESULTATS DEFINITIFS RESUME Combien sommes-nous au Cameroun en novembre § catégorie 2 : les régions dont l’effectif de la population 2005, plus de 18 ans après notre 2e Recensement se situe entre 1 et 2 millions d’habitants. Ce sont Général de la Population et de l’Habitat effectué en avril dans l’ordre d’importance : les régions du Nord- 1987 ? Ouest (1 728 953 habitants), de l’Ouest (1 720 047 Dix-sept millions quatre cent soixante-trois mille habitants), du Nord (1 687 959 habitants) et du Sud- huit cent trente-six (17 463 836) Ouest (1 316 079 habitants) ; § catégorie 3 : les régions ayant chacune moins C’est là une tendance démographique qui confirme le d’un million d’habitants. Ce sont dans l’ordre : les maintien d’un fort potentiel humain dans notre pays, avec régions de l’Adamaoua (884 289 habitants), de l’Est un taux annuel moyen de croissance démographique (771 755 habitants) et du Sud (634 655 habitants). évalué à 2,8 % au cours de la période 1987-2005. Entre le premier recensement effectué en avril 1976, où le D’avril 1987 à novembre 2005, la densité de population Cameroun comptait 7 663 246 habitants et le troisième du Cameroun est passée de 22,6 habitants au kilomètre recensement réalisé en novembre 2005, la population du carré à 37,5. En 2005, cet indicateur connaît de grandes Cameroun a plus que doublé : son effectif a été multiplié variations géographiques : les régions les plus densément par 2,27 précisément. La persistance de ces tendances peuplées sont par ordre d’importance : le Littoral (124 2 2 démographiques fortes, si elles sont maintenues, situera habitants/km ) et l’Ouest (123,8 habitants/km ), tandis l’effectif de la population du Cameroun à 18,9 millions que celles qui le sont le moins, sont : l’Adamaoua 2 2 au 1er janvier 2009, 19,4 millions au 1er janvier 2010 et (13,9 habitants/km ), le Sud (13,4 habitants/km ) et l’Est 2 21,9 millions au 1er janvier 2015. -

Spatial Distribution of the Pit Builders Antlion's Larvae
International Journal of Scientific and Research Publications, Volume 4, Issue 9, September 2014 1 ISSN 2250-3153 Spatial distribution of the pit builders antlion’s larvae (Neuroptera: Myrmeleontidae) in the septentrional regions of Cameroon (Central Africa) Jean Maoge*, Leonard Simon Tinkeu Ngamo**, Bruno Michel*** André Prost **** ** University of Ngaoundéré, Faculty of Science. Department of Biological Sciences P O Box 454 Ngaoundéré Cameroon. ***Entomologist CIRAD UMR CBGP - Campus International de Baillarguet CS 30016 34988 Montferrier sur Lez – France **** Secretary International Association of Neuropterology Rue de l'église F 39320 Loisia- France Abstract- Antlions (Insecta: Neuroptera) are xerophilous insects 1985) [10]. The funnels are a trapping system in fine soil causing adapt to arid conditions that perform some resilent behaviours to prey to the background in the manner of quicksand. In this case, overcome some noxious effects of the global warming. This the agitations of the trapped prey contribute more to swallow it paper focuses on the determination of the diversity of the antlion than to facilitate his escape. in the Soudano-guinean and Soudano-sahelian area of Cameroon The distribution of living organisms is strongly influenced by analyzes the distribution of antlion larvae in these regions. After biotic and abiotic factors. Climate is the main factor responsible 3 years of survey, 3 antlions species dominate in the North of for the distribution of living organisms in the biosphere. Northern Cameroon especially in the dry season. Pits distribution under Cameroon is characterized by a dry climate with a long dry four tropical trees species is irregular, there is higher density of season and low rainfall (Suchel, 1987) [11]. -
Expanded PDF Profile
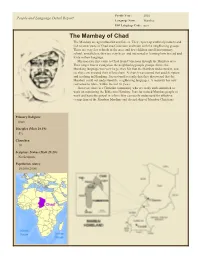
Profile Year: 2001 People and Language Detail Report Language Name: Mambai ISO Language Code: mcs The Mambay of Chad The Mambay are agriculturalist and fishers. They export agricultural products and fish to other parts of Chad and Cameroon and trade with the neighboring groups. There are very few schools in the area, and few children enroll into primary school, nonetheless, they are very keen and interested in learning how to read and write in their language. Missionaries first came to Chad from Cameroon through the Mambay area. Their target was to evangelize the neighboring people groups. Since, the Mundang language was very large, they felt that the Mambay understand it, and so, they concentrated their efforts there. A church was started that used Scripture and teaching in Mundang. It is not until recently that they discovered that the Mambay could not understand the neighboring languages. A majority has now converted to Islam, within the last 30 years. However, there is a Christian community who are ready and committed to work on translating the Bible into Mambay. Pray for trained Mambay people to work and have the gospel in a form they can easily understand for effective evangelism of the Mambay Muslims and discipleship of Mambay Christians. Primary Religion: Islam Disciples (Matt 28.19): 5% Churches: 10 Scripture Status (Matt 28.20): No Scripture Population (date): 10,000 (2000) The Mambay of Chad Item Name Item Note Have They Heard The Gospel? Profile Summary Call Themselves Christian (%) 25 Believe In Jesus As God & Only Savior (%) 5% Prophet/Good Man, But Not God's Son (%) 75 Have Not Heard Who Jesus is (%) ? Believe Jesus Is A Myth (%) 0 Number Of Pastors 10 Comment (Pastors) 10 pastors based on pastor ratio of 1:1,000 and population of 10,000 Number Of Communities 26 villages Number Of Churches 10 Is The Word Of God Translated? No Any Hinderance To Scripture Distribution? Illiteracy rate is quite high. -

Floods and Cholera Epidemic
EPoA Operations Update Cameroon: Floods and Cholera Epidemic DREF Operation no. MDRCM020 GLIDE n° EP-2014-000121-CMR Operations update n° 1 Period covered by this update: 8 September to 5 November, 2014 Operation start date:8 September, 2014 Timeframe: 5 months (End date: 5 February 2015) Operation budget:CHF 308,136 (Initial allocation:CHF 179,304;additional allocation:CHF 128,832) N° of people being assisted: 52,380 (initial number targeted to be assisted was 26,380) Host National Society presence: 25,000 volunteers, more than 50 staff, 58 departmental branches and 250 local branches. Red Cross Red Crescent Movement partners actively involved in the operation: IFRC Other partner organizations actively involved in the operation: Ministry of Public Health, Local Administration Authorities Summary: This operations update extends the operation timeframe by an additional 2 months with an additional budget allocation of CHF 128,832 to; Reinforce public outreach / awareness-raising activities in the six localities, which were included in the EPoA (Bibemi, Figuil, Guider, Mayo-Oulo, Pitoa, and Touboro), in an effort to improve the populations’ sanitation and hygiene practices and thus combat the cholera epidemic in 12 districts (six additional districts) of the North Region of Cameroon Extend these activities into the six localities where new cases have been reported (Garoua I, Garoua II, Gashiga, Golombé, Rey Bouba and Tcholiré). The DREF budget revision will cover the training (on Epidemic Control for Volunteers (ECV) manual); and deployment of an Cameroon RC volunteers offloading NFIs in Bibemi. Photo: IFRC additional 50 volunteers and five supervisors (additional 10 volunteers and one supervisor per locality) in five existing localities (Figuil, Guider, Mayo-Oulo, Pitoa, and Touboro); as well as an additional 130 volunteers and 13 supervisors in the six localities where new cases have been reported (Garoua I, Garoua II, Gashiga, Golombe, Rey Bouba and Tcholiré.