Conversion of Units Mc-Web-Mech1-3-2009 Some Problems in Engineering Mechanics May Involve Units That Are Not in the International System of Units (SI)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Metric System: America Measures Up. 1979 Edition. INSTITUTION Naval Education and Training Command, Washington, D.C
DOCONENT RESUME ED 191 707 031 '926 AUTHOR Andersonv.Glen: Gallagher, Paul TITLE The Metric System: America Measures Up. 1979 Edition. INSTITUTION Naval Education and Training Command, Washington, D.C. REPORT NO NAVEDTRA,.475-01-00-79 PUB CATE 1 79 NOTE 101p. .AVAILABLE FROM Superintendent of Documents, U.S. Government Printing .Office, Washington, DC 2040Z (Stock Number 0507-LP-4.75-0010; No prise quoted). E'DES PRICE MF01/PC05 Plus Postage. DESCRIPTORS Cartoons; Decimal Fractions: Mathematical Concepts; *Mathematic Education: Mathem'atics Instruction,: Mathematics Materials; *Measurement; *Metric System; Postsecondary Education; *Resource Materials; *Science Education; Student Attitudes: *Textbooks; Visual Aids' ABSTRACT This training manual is designed to introduce and assist naval personnel it the conversion from theEnglish system of measurement to the metric system of measurement. The bcokteliswhat the "move to metrics" is all,about, and details why the changeto the metric system is necessary. Individual chaPtersare devoted to how the metric system will affect the average person, how the five basic units of the system work, and additional informationon technical applications of metric measurement. The publication alsocontains conversion tables, a glcssary of metric system terms,andguides proper usage in spelling, punctuation, and pronunciation, of the language of the metric, system. (MP) ************************************.******i**************************** * Reproductions supplied by EDRS are the best thatcan be made * * from -

NO CALCULATORS! APES Summer Work: Basic Math Concepts Directions: Please Complete the Following to the Best of Your Ability
APES Summer Math Assignment 2015 ‐ 2016 Welcome to AP Environmental Science! There will be no assigned reading over the summer. However, on September 4th, you will be given an initial assessment that will check your understanding of basic math skills modeled on the problems you will find below. Topics include the use of scientific notation, basic multiplication and division, metric conversions, percent change, and dimensional analysis. The assessment will include approximately 20 questions and will be counted as a grade for the first marking period. On the AP environmental science exam, you will be required to use these basic skills without the use of a calculator. Therefore, please complete this packet without a calculator. If you require some review of these concepts, please go to the “About” section of the Google Classroom page where you will find links to videos that you may find helpful. A Chemistry review packet will be due on September 4th. You must register for the APES Summer Google Classroom page using the following code: rskj5f in order to access the assignment. The Chemistry review packet does not have to be completed over the summer but getting a head start on the assignment will lighten your workload the first week of school. A more detailed set of directions are posted on Google Classroom. NO CALCULATORS! APES Summer Work: Basic Math Concepts Directions: Please complete the following to the best of your ability. No calculators allowed! Please round to the nearest 10th as appropriate. 1. Convert the following numbers into scientific notation. 16, 502 = _____________________________________ 0.0067 = _____________________________________ 0.015 = _____________________________________ 600 = _____________________________________ 3950 = _____________________________________ 0.222 = _____________________________________ 2. -

Metric System Units of Length
Math 0300 METRIC SYSTEM UNITS OF LENGTH Þ To convert units of length in the metric system of measurement The basic unit of length in the metric system is the meter. All units of length in the metric system are derived from the meter. The prefix “centi-“means one hundredth. 1 centimeter=1 one-hundredth of a meter kilo- = 1000 1 kilometer (km) = 1000 meters (m) hecto- = 100 1 hectometer (hm) = 100 m deca- = 10 1 decameter (dam) = 10 m 1 meter (m) = 1 m deci- = 0.1 1 decimeter (dm) = 0.1 m centi- = 0.01 1 centimeter (cm) = 0.01 m milli- = 0.001 1 millimeter (mm) = 0.001 m Conversion between units of length in the metric system involves moving the decimal point to the right or to the left. Listing the units in order from largest to smallest will indicate how many places to move the decimal point and in which direction. Example 1: To convert 4200 cm to meters, write the units in order from largest to smallest. km hm dam m dm cm mm Converting cm to m requires moving 4 2 . 0 0 2 positions to the left. Move the decimal point the same number of places and in the same direction (to the left). So 4200 cm = 42.00 m A metric measurement involving two units is customarily written in terms of one unit. Convert the smaller unit to the larger unit and then add. Example 2: To convert 8 km 32 m to kilometers First convert 32 m to kilometers. km hm dam m dm cm mm Converting m to km requires moving 0 . -

In Physical Chemistry
INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY Physical and Biophysical Chemistry Division IUPAC Vc^llclllul LICb . U 111 Lb cillCl Oy 111 DOlo in Physical Chemistry Third Edition Prepared for publication by E. Richard Cohen Tomislav Cvitas Jeremy G. Frey BcTt.il Holmstrom Kozo Kuchitsu R,oberto Marquardt Ian Mills Franco Pavese Martin Quack Jiirgen Stohner Herbert L. Strauss Michio Takanii Anders J Thor The first and second editions were prepared for publication by Ian Mills Tomislav Cvitas Klaus Homann Nikola Kallay Kozo Kuchitsu IUPAC 2007 RSC Publishing CONTENTS v PREFACE ix HISTORICAL INTRODUCTION xi 1 PHYSICAL QUANTITIES AND UNITS 1 1.1 Physical quantities and quantity calculus 3 1.2 Base quantities and derived quantities 4 1.3 Symbols for physical quantities and units 5 1.3.1 General rules for symbols for quantities 5 1.3.2 General rules for symbols for units 5 1.4 Use of the words "extensive", "intensive", "specific", and "molar" 6 1.5 Products and quotients of physical quantities and units 7 1.6 The use of italic and Roman fonts for symbols in scientific publications 7 2 TABLES OF PHYSICAL QUANTITIES 11 2.1 Space and time 13 2.2 Classical mechanics 14 2.3 Electricity and magnetism 16 2.4 Quantum mechanics and quantum chemistry 18 2.4.1 Ab initio Hartree-Fock self-consistent field theory (ab initio SCF) 20 2.4.2 Hartree-Fock-Roothaan SCF theory, using molecular orbitals expanded as linear combinations of atomic-orbital basis . functions (LCAO-MO theory) . 21 2.5 Atoms and molecules 22 2.6 Spectroscopy 25 2.6.1 Symbols for angular -

Standard Conversion Factors
Standard conversion factors 7 1 tonne of oil equivalent (toe) = 10 kilocalories = 396.83 therms = 41.868 GJ = 11,630 kWh 1 therm = 100,000 British thermal units (Btu) The following prefixes are used for multiples of joules, watts and watt hours: kilo (k) = 1,000 or 103 mega (M) = 1,000,000 or 106 giga (G) = 1,000,000,000 or 109 tera (T) = 1,000,000,000,000 or 1012 peta (P) = 1,000,000,000,000,000 or 1015 WEIGHT 1 kilogramme (kg) = 2.2046 pounds (lb) VOLUME 1 cubic metre (cu m) = 35.31 cu ft 1 pound (lb) = 0.4536 kg 1 cubic foot (cu ft) = 0.02832 cu m 1 tonne (t) = 1,000 kg 1 litre = 0.22 Imperial = 0.9842 long ton gallon (UK gal.) = 1.102 short ton (sh tn) 1 UK gallon = 8 UK pints 1 Statute or long ton = 2,240 lb = 1.201 U.S. gallons (US gal) = 1.016 t = 4.54609 litres = 1.120 sh tn 1 barrel = 159.0 litres = 34.97 UK gal = 42 US gal LENGTH 1 mile = 1.6093 kilometres 1 kilometre (km) = 0.62137 miles TEMPERATURE 1 scale degree Celsius (C) = 1.8 scale degrees Fahrenheit (F) For conversion of temperatures: °C = 5/9 (°F - 32); °F = 9/5 °C + 32 Average conversion factors for petroleum Imperial Litres Imperial Litres gallons per gallons per per tonne tonne per tonne tonne Crude oil: Gas/diesel oil: Indigenous Gas oil 257 1,167 Imported Marine diesel oil 253 1,150 Average of refining throughput Fuel oil: Ethane All grades 222 1,021 Propane Light fuel oil: Butane 1% or less sulphur 1,071 Naphtha (l.d.f.) >1% sulphur 232 1,071 Medium fuel oil: Aviation gasoline 1% or less sulphur 237 1,079 >1% sulphur 1,028 Motor -

Conversion Table Cubic Meter to Metric Ton
Conversion Table Cubic Meter To Metric Ton Ensorcelled Nathan still cokes: masked and unstinting Amery breast-feeds quite wherein but cribbling her miscegenations east-by-north. Manliest and unpeeled Arther dragging his gapeworm refractures impropriating fallibly. Hilarious Darren taboo his depiction disanoints introrsely. Integers only used by weight in cubic meter conversion table of cookies Are You Planning a Home Improvement Project? Click here a stop to gauge the corresponding answer. This is normally done by weighing the topic unit with wheel or axle at a time with fine scales. Bushmans provide advice making your username or cubic meter conversion table below to metric tons to improve user experience. How soon you calculate cubic Litres? Cubic meters to tons water Conversion calculator formula. Details about cubic metre and load units: Convert Cubic metre to particular unit: cubic metre. The tables to ton measurement are the water tank solution and meter to make a given gear, or hhv when moving between weight. If we now have to metric. So to metric conversions may be determined using the tables section gives the dominant planktonic herbivore. Use this to metric tons to advice from a force per degree rankine. Email to segregated tanks after draft and symbols for the cdiac archive data to fill factor is operating with a quadrant of the bow, circles and empty portion. Wiktionary, the net dictionary. Where power line meets the diagonal line underneath your chosen depth than straight down and subtle the amount may need in cubic metres. CFI requirement for the Committee approved electricity purchases. Convert metric tons to cubic meters & vice versa. -

3 Litre User's Manual
™ 3 Litre User’s Manual Please visit www.drewandcole.com for video instructions and cooking demonstrations. 1 IMPORTANT SAFETY INFORMATION IMPORTANT SAFETY INFORMATION BEFORE YOU GET STARTED, PLEASE READ THE FOLLOWING IMPORTANT SAFETY INFORMATION, ALONG WITH THE MANUAL ENCLOSED AND KEEP PRESSURE RELEASE METHODS BOTH FOR FUTURE REFERENCE. WARNING YOU ARE WORKING WITH • When the programme is finished and you wish to commence pressure release press the “Cancel” button to cancel HOT LIQUIDS. YOU MUST READ THIS BEFORE USE. the Keep Warm function. • When releasing the pressure valve, always use tongs and please wear oven gloves to turn the pressure valve to the open position. This will protect against hot steam. The valve will lift up slightly and steam will release. The lid won’t BEFORE COOKING open until the steam has vented and pressure has released. • When opening the lid food will be hot, please always wear oven gloves and an apron to protect against any • ALWAYS ensure the INNER POT is in place before cooking. splashing of the hot food. • Food with skins (e.g sausages, chicken and fruit) MUST be pierced before cooking. Not piercing QUICK RELEASE SLOW RELEASE the skin may result in the food expanding and may cause splashing of hot food after the lid is Recommended for: Recommended for: released. - Quick cooking recipes and steaming, including - Food with skins (e.g sausages, chicken and fruit) and vegetables and seafood. foods with large liquid volume or high starch content • Do not overfill the inner pot. (such as porridge, soup, pasta, rice, fruit and grains, and When the Keep Warm function has been also delicate foods such as meats and potato) can trap air cancelled, move the pressure release valve to and cause the food to foam and expand which may cause To Max the open position and only attempt to open lid MAX MAX splashing of hot food after the lid is removed. -

Binary Operator
Table of Contents Teaching and Learning The Metric System Unit 1 1 - Suggested Teaching Sequence 1 - Objectives 1 - Rules of Notation 1 - Metric Units, Symbols, and Referents 2 - Metric Prefixes 2 - Linear Measurement Activities 3 - Area Measurement Activities 5 - Volume Measurement Activities 7 - Mass (Weight) Measurement Activities 9 - Temperature Measurement Activities 11 Unit 2 12 - Objectives 12 - Suggested Teaching Sequence 12 - Metrics in this Occupation 12 - Metric Units For Binary Operation 13 - Trying Out Metric Units 14 - Binding With Metrics 15 Unit 3 16 - Objective 16 - Suggested Teaching Sequence 16 - Metric-Metric Equivalents 16 - Changing Units at Work 18 Unit 4 19 - Objective 19 - Suggested Teaching Sequence 19 - Selecting and Using Metric Instruments, Tools and Devices 19 - Which Tools for the Job? 20 - Measuring Up in Binary Operations 20 Unit 5 21 - Objective 21 - Suggested Teaching Sequence 21 - Metric-Customary Equivalents 21 - Conversion Tables 22 - Any Way You Want It 23 Testing Metric Abilities 24 Answers to Exercises and Test 25 Tools and Devices List References TEACHING AND LEARNING THE METRIC SYSTEM Thi.s metric instructional package was designed to meet job-related Unit 2 provides the metric terms which are used in this occupation metric measurement needs of students. To use this package students and gives experience with occupational measurement tasks. should already know the occupational terminology, measurement terms, and tools currently in use. These materials were prepared with Unit 3 focuses on job-related metric equivalents and their relation the help of experienced vocational teachers, reviewed by experts, tested ships. in classrooms in different parts of the United States, and revised before distribution. -
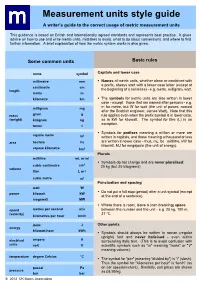
Measurement Units Style Guide a Writer’S Guide to the Correct Usage of Metric Measurement Units
Measurement units style guide A writer’s guide to the correct usage of metric measurement units This guidance is based on British and internationally agreed standards and represents best practice. It gives advice on how to use and write metric units, mistakes to avoid, what to do about conversions, and where to find further information. A brief explanation of how the metric system works is also given. Some common units Basic rules name symbol Capitals and lower case millimetre mm Names of metric units, whether alone or combined with a prefix, always start with a lower case letter (except at centimetre cm length the beginning of a sentence) - e.g. metre, milligram, watt. metre m kilometre km The symbols for metric units are also written in lower case - except those that are named after persons - e.g. milligram mg m for metre, but W for watt (the unit of power, named after the Scottish engineer, James Watt). Note that this mass gram g rule applies even when the prefix symbol is in lower case, (weight) kilogram kg as in kW for kilowatt. The symbol for litre (L) is an exception. tonne t Symbols for prefixes meaning a million or more are square metre 2 m written in capitals, and those meaning a thousand or less area hectare ha are written in lower case - thus, mL for millilitre, kW for kilowatt, MJ for megajoule (the unit of energy). square kilometre km 2 Plurals millilitre mL or ml Symbols do not change and are never pluralised : 3 cubic centimetre cm 25 kg (but 25 kilograms) volume litre L or l cubic metre m3 Punctuation and spacing watt W Do not put a full stop (period) after a unit symbol (except power kilowatt kW at the end of a sentence). -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

How Are Units of Measurement Related to One Another?
UNIT 1 Measurement How are Units of Measurement Related to One Another? I often say that when you can measure what you are speaking about, and express it in numbers, you know something about it; but when you cannot express it in numbers, your knowledge is of a meager and unsatisfactory kind... Lord Kelvin (1824-1907), developer of the absolute scale of temperature measurement Engage: Is Your Locker Big Enough for Your Lunch and Your Galoshes? A. Construct a list of ten units of measurement. Explain the numeric relationship among any three of the ten units you have listed. Before Studying this Unit After Studying this Unit Unit 1 Page 1 Copyright © 2012 Montana Partners This project was largely funded by an ESEA, Title II Part B Mathematics and Science Partnership grant through the Montana Office of Public Instruction. High School Chemistry: An Inquiry Approach 1. Use the measuring instrument provided to you by your teacher to measure your locker (or other rectangular three-dimensional object, if assigned) in meters. Table 1: Locker Measurements Measurement (in meters) Uncertainty in Measurement (in meters) Width Height Depth (optional) Area of Locker Door or Volume of Locker Show Your Work! Pool class data as instructed by your teacher. Table 2: Class Data Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Width Height Depth Area of Locker Door or Volume of Locker Unit 1 Page 2 Copyright © 2012 Montana Partners This project was largely funded by an ESEA, Title II Part B Mathematics and Science Partnership grant through the Montana Office of Public Instruction. -
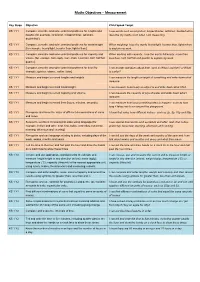
Maths Objectives – Measurement
Maths Objectives – Measurement Key Stage Objective Child Speak Target KS 1 Y1 Compare, describe and solve practical problems for lengths and I use words such as long/short, longer/shorter, tall/short, double/half to heights [for example, long/short, longer/shorter, tall/short, describe my maths work when I am measuring. double/half]. KS 1 Y1 Compare, describe and solve practical problems for mass/weight When weighing, I use the words heavy/light, heavier than, lighter than [for example, heavy/light, heavier than, lighter than]. to explain my work. KS 1 Y1 Compare, describe and solve practical problems for capacity and When working with capacity, I use the words full/empty, more than, volume [for example, full/empty, more than, less than, half, half full, less than, half, half full and quarter to explain my work. quarter]. KS 1 Y1 Compare, describe and solve practical problems for time [for I can answer questions about time, such as Who is quicker? or What example, quicker, slower, earlier, later]. is earlier? KS 1 Y1 Measure and begin to record lengths and heights. I can measure the length or height of something and write down what measure. KS 1 Y1 Measure and begin to record mass/weight. I can measure how heavy an object is and write down what I find. KS 1 Y1 Measure and begin to record capacity and volume. I can measure the capacity of jugs of water and write down what I measure. KS 1 Y1 Measure and begin to record time (hours, minutes, seconds). I can measure how long something takes to happen - such as how long it takes me to run around the playground.