Differential Responses to Α-Pinene of Two Horntail Wasps, Urocerus Antennatus and Xeris Spectrum (Hymenoptera: Siricidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mycelial Compatibility in Amylostereum Areolatum
Mycelial compatibility in Amylostereum areolatum Magrieta Aletta van der Nest © University of Pretoria © University of Pretoria Mycelial compatibility in Amylostereum areolatum by Magrieta Aletta van der Nest Promotor: Prof. B.D. Wingfield Co-promotor: Prof. M.J. Wingfield Prof. B. Slippers Prof. J. Stenlid Submitted in partial fulfilment of the requirements for the degree of Philosophiae Doctor in the Faculty of Natural and Agricultural Sciences, Department of Genetics at the University of Pretoria. September 2010 © University of Pretoria DECLARATION I, Magrieta Aletta van der Nest, declare that this thesis, which I hereby submit for the degree Philosophiae Doctor at the University of Pretoria, is my own work and has not been submitted by me at this or any other tertiary institution. SIGNATURE: DATE: © University of Pretoria TABLE OF CONTENTS ACKNOWLEDGEMENTS ................................................................................................... i PREFACE ............................................................................................................................... ii CHAPTER 1 ........................................................................................................................... 1 LITERATURE REVIEW: The molecular basis of vegetative incompatibility in fungi, with specific reference to Basidiomycetes CHAPTER 2 ......................................................................................................................... 44 Genetics of Amylostereum species associated with Siricidae -

The Ecology, Behavior, and Biological Control Potential of Hymenopteran Parasitoids of Woodwasps (Hymenoptera: Siricidae) in North America
REVIEW:BIOLOGICAL CONTROL-PARASITOIDS &PREDATORS The Ecology, Behavior, and Biological Control Potential of Hymenopteran Parasitoids of Woodwasps (Hymenoptera: Siricidae) in North America 1 DAVID R. COYLE AND KAMAL J. K. GANDHI Daniel B. Warnell School of Forestry and Natural Resources, University of Georgia, Athens, GA 30602 Environ. Entomol. 41(4): 731Ð749 (2012); DOI: http://dx.doi.org/10.1603/EN11280 ABSTRACT Native and exotic siricid wasps (Hymenoptera: Siricidae) can be ecologically and/or economically important woodboring insects in forests worldwide. In particular, Sirex noctilio (F.), a Eurasian species that recently has been introduced to North America, has caused pine tree (Pinus spp.) mortality in its non-native range in the southern hemisphere. Native siricid wasps are known to have a rich complex of hymenopteran parasitoids that may provide some biological control pressure on S. noctilio as it continues to expand its range in North America. We reviewed ecological information about the hymenopteran parasitoids of siricids in North America north of Mexico, including their distribution, life cycle, seasonal phenology, and impacts on native siricid hosts with some potential efÞcacy as biological control agents for S. noctilio. Literature review indicated that in the hymenop- teran families Stephanidae, Ibaliidae, and Ichneumonidae, there are Þve genera and 26 species and subspecies of native parasitoids documented from 16 native siricids reported from 110 tree host species. Among parasitoids that attack the siricid subfamily Siricinae, Ibalia leucospoides ensiger (Norton), Rhyssa persuasoria (L.), and Megarhyssa nortoni (Cresson) were associated with the greatest number of siricid and tree species. These three species, along with R. lineolata (Kirby), are the most widely distributed Siricinae parasitoid species in the eastern and western forests of North America. -

New Data on the Occurence of Horntails in Poland (Hymenoptera, Symphyta: Siricidae)
Available online at www.worldscientificnews.com WSN 136 (2019) 241-246 EISSN 2392-2192 SHORT COMMUNICATION New data on the occurence of horntails in Poland (Hymenoptera, Symphyta: Siricidae) Borowski Jerzy1,*, Marczak Dawid2, Szawaryn Karol3, Kwiatkowski Adam4, Cieślak Rafał1, Buchholz Lech5 1Department of Forest Protection and Ecology, Warsaw University of Life Sciences, ul. Nowoursynowska 159/34, 02-776 Warsaw, Poland 2Kampinos National Park, ul. Tetmajera 38, 05-080 Izabelin, Poland University of Ecology and Management in Warsaw, ul. Olszewska 12, 00-792 Warsaw, Poland 3Museum and Institute of Zoology, Polish Academy of Sciences, ul. Wilcza 64, 00-679 Warsaw, Poland 4Regional Directorate of the State Forests in Białystok, ul. Lipowa 51, 15-424 Białystok, Poland Bialystok University of Technology, ul. Wiejska 54A, 15-351 Białystok, Poland 5Świętokrzyski National Park, ul. Suchedniowska 4, 26-010 Bodzentyn, Poland *E-mail address: [email protected] ABSTRACT The paper presents new data on the occurrence of 6 sawflies species of the Siricidae family, on the territory of Poland. New faunistic data was supplemented with elements of bionomics and information on geographical distribution of particular species. Keywords: Hymenoptera, Symphyta, sawflies, Siricidae, funistic data, Poland ( Received 28 September 2019; Accepted 10 October 2019; Date of Publication 15 October 2019 ) World Scientific News 136 (2019) 241-246 INTRODUCTION Horntails (Siricidae) is one of Symphyta families rather poor in species number, represented in Poland by 10 species (Borowski & al. 2019). Most of them are trophically connected with coniferous trees, while only the species of Tremex Jurine genus live on deciduous trees. Horntails are classified as xylophages, i.e. the insects whose total development, from egg laying to the occurrence of imagines, takes place in wood. -

THE SIRICID WOOD WASPS of CALIFORNIA (Hymenoptera: Symphyta)
Uroce r us californ ic us Nott on, f ema 1e. BULLETIN OF THE CALIFORNIA INSECT SURVEY VOLUME 6, NO. 4 THE SIRICID WOOD WASPS OF CALIFORNIA (Hymenoptera: Symphyta) BY WOODROW W. MIDDLEKAUFF (Department of Entomology and Parasitology, University of California, Berkeley) UNIVERSITY OF CALIFORNIA PRESS BERKELEY AND LOS ANGELES 1960 BULLETIN OF THE CALIFORNIA INSECT SURVEY Editors: E. G: Linsley, S. B. Freeborn, P. D. Hurd, R. L. Usinget Volume 6, No. 4, pp. 59-78, plates 4-5, frontis. Submitted by editors October 14, 1958 Issued April 22, 1960 Price, 50 cents UNIVERSITY OF CALIFORNIA PRESS BERKELEY AND LOS ANGELES CALIFORNIA CAMBRIDGE UNIVERSITY PRESS LONDON, ENGLAND PRINTED BY OFFSET IN THE UNITED STATES OF AMERICA THE SIRICID WOOD WASPS OF CALIFORNIA (Hymenoptera: Symphyta) BY WOODROW W. MIDDLEKAUFF INTRODUCTION carpeting. Their powerful mandibles can even cut through lead sheathing. The siricid wood wasps are fairly large, cylin- These insects are widely disseminated by drical insects; usually 20 mm. or more in shipments of infested lumber or timber, and length with the head, thorax, and abdomen of the adults may not emerge until several years equal width. The antennae are long and fili- have elapsed. Movement of this lumber and form, with 14 to 30 segments. The tegulae are timber tends to complicate an understanding minute. Jn the female the last segment of the of the normal distribution pattern of the spe- abdomen bears a hornlike projection called cies. the cornus (fig. 8), whose configuration is The Nearctic species in the family were useful for taxonomic purposes. This distinc- monographed by Bradley (1913). -

A Review of the Genus Amylostereum and Its Association with Woodwasps
70 South African Journal of Science 99, January/February 2003 Review Article A review of the genus Amylostereum and its association with woodwasps B. Slippers , T.A. Coutinho , B.D. Wingfield and M.J. Wingfield Amylostereum.5–7 Today A. chailletii, A. areolatum and A. laevigatum are known to be symbionts of a variety of woodwasp species.7–9 A fascinating symbiosis exists between the fungi, Amylostereum The relationship between Amylostereum species and wood- chailletii, A. areolatum and A. laevigatum, and various species of wasps is highly evolved and has been shown to be obligatory siricid woodwasps. These intrinsic symbioses and their importance species-specific.7–10 The principal advantage of the relationship to forestry have stimulated much research in the past. The fungi for the fungus is that it is spread and effectively inoculated into have, however, often been confused or misidentified. Similarly, the new wood, during wasp oviposition.11,12 In turn the fungus rots phylogenetic relationships of the Amylostereum species with each and dries the wood, providing a suitable environment, nutrients other, as well as with other Basidiomycetes, have long been unclear. and enzymes that are important for the survival and develop- Recent studies based on molecular data have given new insight ment of the insect larvae (Fig. 1).13–17 into the taxonomy and phylogeny of the genus Amylostereum. The burrowing activity of the siricid larvae and rotting of the Molecular sequence data show that A. areolatum is most distantly wood by Amylostereum species makes this insect–fungus symbio- related to other Amylostereum species. Among the three other sis potentially harmful to host trees, which include important known Amylostereum species, A. -

Re-Thinking the Classification of Corticioid Fungi
mycological research 111 (2007) 1040–1063 journal homepage: www.elsevier.com/locate/mycres Re-thinking the classification of corticioid fungi Karl-Henrik LARSSON Go¨teborg University, Department of Plant and Environmental Sciences, Box 461, SE 405 30 Go¨teborg, Sweden article info abstract Article history: Corticioid fungi are basidiomycetes with effused basidiomata, a smooth, merulioid or Received 30 November 2005 hydnoid hymenophore, and holobasidia. These fungi used to be classified as a single Received in revised form family, Corticiaceae, but molecular phylogenetic analyses have shown that corticioid fungi 29 June 2007 are distributed among all major clades within Agaricomycetes. There is a relative consensus Accepted 7 August 2007 concerning the higher order classification of basidiomycetes down to order. This paper Published online 16 August 2007 presents a phylogenetic classification for corticioid fungi at the family level. Fifty putative Corresponding Editor: families were identified from published phylogenies and preliminary analyses of unpub- Scott LaGreca lished sequence data. A dataset with 178 terminal taxa was compiled and subjected to phy- logenetic analyses using MP and Bayesian inference. From the analyses, 41 strongly Keywords: supported and three unsupported clades were identified. These clades are treated as fam- Agaricomycetes ilies in a Linnean hierarchical classification and each family is briefly described. Three ad- Basidiomycota ditional families not covered by the phylogenetic analyses are also included in the Molecular systematics classification. All accepted corticioid genera are either referred to one of the families or Phylogeny listed as incertae sedis. Taxonomy ª 2007 The British Mycological Society. Published by Elsevier Ltd. All rights reserved. Introduction develop a downward-facing basidioma. -

Amylostereum Laevigatum Associated with the Japanese Horntail, Urocerusjaponicus
421 Mycoscience 38: 421-427, 1997 Amylostereum laevigatum associated with the Japanese horntail, Urocerusjaponicus Masanobu Tabata 1~ and Yasuhisa Abe z) 1~ Shikoku Research Center, Forestry and Forest Products Research Institute, 915, Asakura-tei, Kochi 780, Japan 2~ Forestry and Forest Products Research Institute, P. O. Box 16, Tsukuba, Ibaraki 305, Japan Accepted for publication 30 October 1997 The fungus associated with the Japanese horntail, Urocerus japonicus, in Kochi, Kagawa and Ehime Prefectures was studied. Cultures isolated from the mycangia of 113 adult females of the horntail showed the same cultural charac- teristics. Four of basidiocarps found on felled logs of Cryptomeriajaponica were identified as Amylostereumlaevigatum based on morphological characteristics. This was the first record of A. laevigatum from Japan. The cultures isolated from the basidiocarps had the same cultural characteristics as those from the mycangia of U.japonicus. One mycangial isolate produced basidiocarps on artificially inoculated stem segments of Cr. japonica after a 6-too incubation and was identified as A. laevigatum. One isolate from the basidiocarps of A. laevigatum and one from the mycangium of U. japonicus were artificially inoculated into five trees each of Chamaecyparis obtusa and Cr. japonica. The wood of all in- oculated trees showed discoloration, with no difference in shape and pattern of discoloration between the two isolates. The inoculated fungi were reisolated from the areas of discoloration in the inoculated trees. Key Words- Amylostereum laevigatum; Japanese horntail; symbiont; Urocerusjaponicus; wood discoloration. Of the five recorded species of Amylostereum (Chamuris, quired to identify the symbionts exactly by teleomorph, 1988), onlyA, areolatum (Fr.: Fr.) Boidin and A. -

Technical Series, No
' ' Technical Series, No. 20, Part II. U. S. DEPARTMENT OF AGRICULTURE, BXJRE^TJ OK' TClSrTOM:OIL.OG^Y. L, 0. HOWARD, Entomologist and Chief of Bureau. TECHNICAL PAPERS ON MISCELLANEOUS .FOREST INSECTS. II. THE GENOTYPES OF THE SAWFLIES AND WOODWASPS, OR THE SUPERFAMILY TENTHKEDINOIDEA. S. A. ROHWER, Agent and Expert. Issued M.\rch 4, 1911. WASHINGTON: GOVERNMENT PRINTING OFFICE. 1911. Technical Series, No. 20, Part II. U. S. DEPARTMENT OF AGRICULTURE. L. 0. HOWARD, Entomologist and Chief of Bureau. TECHNICAL PAPERS ON MISCELLANEOUS FOREST INSECTS. II. THE GENOTYPES OF THE SAWFLIES AND WOODWASPS, OR THE SUPERFAMILY TENTHREDINOIDEA. BY S. A. ROHWER, Agent and Expert. Issued Makch 4, 1911. WASHINGTON: GOVERNMENT PRINTING OFFICE. 1911. B UREA U OF ENTOMOLOGY. L. O. Howard, Entomologist and Chief of Bureau. C. L. Marlatt, Entomologist and Acting Chief in Absence of Chief. R. S. Clifton, Executive Assistant. W. F. Tastet, Chief Clerk. F. H. Chittenden, in charge of truck crop and stored product insect investigations. A. D. Hopkins, in charge offorest insect investigations. W. D. Hunter, in charge of southern field crop insect investigations. F. M. Webster, in charge of cereal and forage insect investigations. A. L. Quaintance, in charge of deciduous fruit insect investigations. E. F. Phillips, in charge of bee culture. D. M. Rogers, in charge of preventing spread of moths, field -work. RoLLA P. Currie, in charge of editorial work. Mabel Colcord, librarian. , Forest Insect Investigations. A. D. Hopkins, in charge. H. E. Burke, J. L. Webb, Josef Brunner, S. A. Rohwer, T. E. Snyder, W. D. Edmonston, W. B. Turner, agents and experts. -

Commodity Risk Assessment of Black Pine (Pinus Thunbergii Parl.) Bonsai from Japan
SCIENTIFIC OPINION ADOPTED: 28 March 2019 doi: 10.2903/j.efsa.2019.5667 Commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan EFSA Panel on Plant Health (EFSA PLH Panel), Claude Bragard, Katharina Dehnen-Schmutz, Francesco Di Serio, Paolo Gonthier, Marie-Agnes Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas-Cortes, Stephen Parnell, Philippe Lucien Reignault, Hans-Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen, Lucia Zappala, Andrea Battisti, Anna Maria Vettraino, Renata Leuschner, Olaf Mosbach-Schulz, Maria Chiara Rosace and Roel Potting Abstract The EFSA Panel on Plant health was requested to deliver a scientific opinion on how far the existing requirements for the bonsai pine species subject to derogation in Commission Decision 2002/887/EC would cover all plant health risks from black pine (Pinus thunbergii Parl.) bonsai (the commodity defined in the EU legislation as naturally or artificially dwarfed plants) imported from Japan, taking into account the available scientific information, including the technical information provided by Japan. The relevance of an EU-regulated pest for this opinion was based on: (a) evidence of the presence of the pest in Japan; (b) evidence that P. thunbergii is a host of the pest and (c) evidence that the pest can be associated with the commodity. Sixteen pests that fulfilled all three criteria were selected for further evaluation. The relevance of other pests present in Japan (not regulated in the EU) for this opinion was based on (i) evidence of the absence of the pest in the EU; (ii) evidence that P. -

Prospecting Russula Senecis: a Delicacy Among the Tribes of West Bengal
Prospecting Russula senecis: a delicacy among the tribes of West Bengal Somanjana Khatua, Arun Kumar Dutta and Krishnendu Acharya Molecular and Applied Mycology and Plant Pathology Laboratory, Department of Botany, University of Calcutta, Kolkata, West Bengal, India ABSTRACT Russula senecis, a worldwide distributed mushroom, is exclusively popular among the tribal communities of West Bengal for food purposes. The present study focuses on its reliable taxonomic identification through macro- and micro-morphological features, DNA barcoding, confirmation of its systematic placement by phylogenetic analyses, myco-chemicals and functional activities. For the first time, the complete Internal Transcribed Spacer region of R. senecis has been sequenced and its taxo- nomic position within subsection Foetentinae under series Ingratae of the subgen. Ingratula is confirmed through phylogenetic analysis. For exploration of its medic- inal properties, dried basidiocarps were subjected for preparation of a heat stable phenol rich extract (RusePre) using water and ethanol as solvent system. The an- tioxidant activity was evaluated through hydroxyl radical scavenging (EC50 5 µg/ml), chelating ability of ferrous ion (EC50 0.158 mg/ml), DPPH radical scavenging (EC50 1.34 mg/ml), reducing power (EC50 2.495 mg/ml) and total antioxidant activity methods (13.44 µg ascorbic acid equivalent/mg of extract). RusePre exhibited an- timicrobial potentiality against Listeria monocytogenes, Bacillus subtilis, Pseudomonas aeruginosa and Staphylococcus aureus. Furthermore, diVerent parameters were tested to investigate its chemical composition, which revealed the presence of appreciable quantity of phenolic compounds, along with carotenoids and ascorbic acid. HPLC- UV fingerprint indicated the probable existence of at least 13 phenolics, of which 10 were identified (pyrogallol > kaempferol > quercetin > chlorogenic acid > ferulic Submitted 29 November 2014 acid, cinnamic acid > vanillic acid > salicylic acid > p-coumaric acid > gallic acid). -
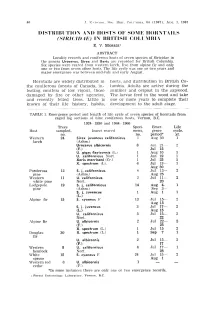
" ANI) HOSTS of SO"IE 1I0HNTAILS (SI Riel F)A F,,) in BH ITISII COLL'i BL\
00 .T . '·;'TO \lO I.. Se .... BIUT. CO I.I · \""A. 64 (1967)' A UG. 1. 1967 OISTHIBlTIO," ANI) HOSTS OF SO"IE 1I0HNTAILS (SI Riel f)A f,,) IN BH ITISII COLL'I BL\ E. V. MORRIS I ABSTRACT Locality recol'ds and con iferous hosts of seven species of Siricidae in the genera Urocerus, Sirex and Xeris are recorded for British Columbia. Six species were reared from western larch. fi ve from alpine fir and only one or two from seven other hosts. The life cycle was one or two years and major emergence was betw('('n mid-July ami early August. Horntails are widely dis tributed in hosts, and dis tribution in British Co the coniferous forests of Ca nada, in lumbia. Adults are active during the festing conifers of low vigour, those s ummer a nd ovipost in the sapwood. damaged by fire or other agencies, The la rvae fe ed in the wood and take and recently felled trees . Little is one or more years to complete their known of their life history, habits, development to the adult stage. TABLE 1. Emergence period and length of life cycle of sc' ven species of horn tails from caged log sections of nine coniferous hosts, Vernon, B.C. 1924 - 1930 and 1964 - 1966 Trees Speci Emer Life Host sampled, Insect reared mens, gence cycle, no. no. period* yr. Weste rn 24 Sirex iuvencus californicus 1 Aug 30 1 larch (Ashm. ) Urocerus albicornis 8 .Jun 21 ~ 2 (F.) Jul 15 U. gigas flavicornis (L.) 1 Aug 10 1 U. -

And Type the TITLE of YOUR WORK in All
TRAPPING METHODS FOR LARGE WOODBORING INSECTS IN SOUTHEASTERN U.S. FORESTS by BRITTANY FRANCES BARNES (Under the Direction of Kamal J.K Gandhi) ABSTRACT Large woodboring insects (Buprestidae, Cerambycidae, Elateridae, and Siricidae) are ecologically and/or economically important in the southeastern U.S. forests. Surveys are conducted annually in the region to monitor populations of native and exotic woodboring beetle species. My research objectives were as follows: 1) to assess the efficacy of various trapping techniques for surveying of native siricids (woodwasps) and their hymenopteran parasitoids in southeastern pine (Pinus spp.) stands; and 2) to determine the effect of lure placement on modified funnel traps on capturing efficiency of large woodboring beetles. Creating trap-logs at the peak flight of siricids (early November) and using fresh pine billets with an intercept panel trap were the most efficient methods for trapping native siricids and their hymenopteran parasitoids. A modified funnel trap with lures hanging on the inside of the trap maximized the catches and diversity of cerambycid and elaterid beetles. INDEX WORDS: Buprestidae, Cerambycidae, Elateridae, hymenopteran parasitoids, Pinus spp., semiochemical lures, Siricidae, Southeastern U.S., trapping, woodboring insects TRAPPING METHODS FOR LARGE WOODBORING INSECTS IN SOUTHEASTERN U.S. FORESTS by BRITTANY FRANCES BARNES BSFR., University of Georgia, 2009 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE ATHENS, GEORGIA 2012 © 2012 Brittany Frances Barnes All Rights Reserved TRAPPING METHODS FOR LARGE WOODBORING INSECTS IN THE SOUTHEASTERN U.S. FORESTS by BRITTANY FRANCES BARNES Major Professor: Kamal J.K Gandhi Committee: Jeffrey F.D.