The Potential Global Distribution of Sirex Juvencus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ecology, Behavior, and Biological Control Potential of Hymenopteran Parasitoids of Woodwasps (Hymenoptera: Siricidae) in North America
REVIEW:BIOLOGICAL CONTROL-PARASITOIDS &PREDATORS The Ecology, Behavior, and Biological Control Potential of Hymenopteran Parasitoids of Woodwasps (Hymenoptera: Siricidae) in North America 1 DAVID R. COYLE AND KAMAL J. K. GANDHI Daniel B. Warnell School of Forestry and Natural Resources, University of Georgia, Athens, GA 30602 Environ. Entomol. 41(4): 731Ð749 (2012); DOI: http://dx.doi.org/10.1603/EN11280 ABSTRACT Native and exotic siricid wasps (Hymenoptera: Siricidae) can be ecologically and/or economically important woodboring insects in forests worldwide. In particular, Sirex noctilio (F.), a Eurasian species that recently has been introduced to North America, has caused pine tree (Pinus spp.) mortality in its non-native range in the southern hemisphere. Native siricid wasps are known to have a rich complex of hymenopteran parasitoids that may provide some biological control pressure on S. noctilio as it continues to expand its range in North America. We reviewed ecological information about the hymenopteran parasitoids of siricids in North America north of Mexico, including their distribution, life cycle, seasonal phenology, and impacts on native siricid hosts with some potential efÞcacy as biological control agents for S. noctilio. Literature review indicated that in the hymenop- teran families Stephanidae, Ibaliidae, and Ichneumonidae, there are Þve genera and 26 species and subspecies of native parasitoids documented from 16 native siricids reported from 110 tree host species. Among parasitoids that attack the siricid subfamily Siricinae, Ibalia leucospoides ensiger (Norton), Rhyssa persuasoria (L.), and Megarhyssa nortoni (Cresson) were associated with the greatest number of siricid and tree species. These three species, along with R. lineolata (Kirby), are the most widely distributed Siricinae parasitoid species in the eastern and western forests of North America. -

New Data on the Occurence of Horntails in Poland (Hymenoptera, Symphyta: Siricidae)
Available online at www.worldscientificnews.com WSN 136 (2019) 241-246 EISSN 2392-2192 SHORT COMMUNICATION New data on the occurence of horntails in Poland (Hymenoptera, Symphyta: Siricidae) Borowski Jerzy1,*, Marczak Dawid2, Szawaryn Karol3, Kwiatkowski Adam4, Cieślak Rafał1, Buchholz Lech5 1Department of Forest Protection and Ecology, Warsaw University of Life Sciences, ul. Nowoursynowska 159/34, 02-776 Warsaw, Poland 2Kampinos National Park, ul. Tetmajera 38, 05-080 Izabelin, Poland University of Ecology and Management in Warsaw, ul. Olszewska 12, 00-792 Warsaw, Poland 3Museum and Institute of Zoology, Polish Academy of Sciences, ul. Wilcza 64, 00-679 Warsaw, Poland 4Regional Directorate of the State Forests in Białystok, ul. Lipowa 51, 15-424 Białystok, Poland Bialystok University of Technology, ul. Wiejska 54A, 15-351 Białystok, Poland 5Świętokrzyski National Park, ul. Suchedniowska 4, 26-010 Bodzentyn, Poland *E-mail address: [email protected] ABSTRACT The paper presents new data on the occurrence of 6 sawflies species of the Siricidae family, on the territory of Poland. New faunistic data was supplemented with elements of bionomics and information on geographical distribution of particular species. Keywords: Hymenoptera, Symphyta, sawflies, Siricidae, funistic data, Poland ( Received 28 September 2019; Accepted 10 October 2019; Date of Publication 15 October 2019 ) World Scientific News 136 (2019) 241-246 INTRODUCTION Horntails (Siricidae) is one of Symphyta families rather poor in species number, represented in Poland by 10 species (Borowski & al. 2019). Most of them are trophically connected with coniferous trees, while only the species of Tremex Jurine genus live on deciduous trees. Horntails are classified as xylophages, i.e. the insects whose total development, from egg laying to the occurrence of imagines, takes place in wood. -

THE SIRICID WOOD WASPS of CALIFORNIA (Hymenoptera: Symphyta)
Uroce r us californ ic us Nott on, f ema 1e. BULLETIN OF THE CALIFORNIA INSECT SURVEY VOLUME 6, NO. 4 THE SIRICID WOOD WASPS OF CALIFORNIA (Hymenoptera: Symphyta) BY WOODROW W. MIDDLEKAUFF (Department of Entomology and Parasitology, University of California, Berkeley) UNIVERSITY OF CALIFORNIA PRESS BERKELEY AND LOS ANGELES 1960 BULLETIN OF THE CALIFORNIA INSECT SURVEY Editors: E. G: Linsley, S. B. Freeborn, P. D. Hurd, R. L. Usinget Volume 6, No. 4, pp. 59-78, plates 4-5, frontis. Submitted by editors October 14, 1958 Issued April 22, 1960 Price, 50 cents UNIVERSITY OF CALIFORNIA PRESS BERKELEY AND LOS ANGELES CALIFORNIA CAMBRIDGE UNIVERSITY PRESS LONDON, ENGLAND PRINTED BY OFFSET IN THE UNITED STATES OF AMERICA THE SIRICID WOOD WASPS OF CALIFORNIA (Hymenoptera: Symphyta) BY WOODROW W. MIDDLEKAUFF INTRODUCTION carpeting. Their powerful mandibles can even cut through lead sheathing. The siricid wood wasps are fairly large, cylin- These insects are widely disseminated by drical insects; usually 20 mm. or more in shipments of infested lumber or timber, and length with the head, thorax, and abdomen of the adults may not emerge until several years equal width. The antennae are long and fili- have elapsed. Movement of this lumber and form, with 14 to 30 segments. The tegulae are timber tends to complicate an understanding minute. Jn the female the last segment of the of the normal distribution pattern of the spe- abdomen bears a hornlike projection called cies. the cornus (fig. 8), whose configuration is The Nearctic species in the family were useful for taxonomic purposes. This distinc- monographed by Bradley (1913). -

The Sirex Woodwasp, Sirex Noctilio: Pest in North America May Be the Ecology, Potential Impact, and Management in the Southeastern U.S
SREF-FH-003 June 2016 woodwasp has not become a major The Sirex woodwasp, Sirex noctilio: pest in North America may be the Ecology, Potential Impact, and Management in the Southeastern U.S. many insects that are competitors or natural enemies. Some of these insects compete for resources AUTHORED BY: LAUREL J. HAAVIK AND DAVID R. COYLE (e.g. native woodwasps, bark and ambrosia beetles, and longhorned beetles) while others (e.g.parasitoids) are natural enemies and use Sirex woodwasp larvae as hosts. However, should the Sirex woodwasp arrive in the southeastern U.S., with its abundant pine plantations and areas of natural pine, this insect could easily be a major pest for the region. Researchers have monitored and tracked Sirex woodwasp populations since its discovery in North America. The most common detection tool is a flight intercept trap (Fig. 2a) baited with a synthetic chemical lure that consists of pine scents (70% α-pinene, 30% β-pinene) or actual pine branches (Fig. 2b). Woodwasps are attracted to the odors given off by the lure or cut pine branches, and as they fly toward the scent they collide with the sides of the trap and drop Figure 1. The high density of likely or confirmed pine (Pinus spp.) hosts of the Sirex woodwasp suggests the southeastern U.S. may be heavily impacted should this non-native insect become into the collection cup at the bottom. established in this region. The collection cup is usually filled with a liquid (e.g. propylene glycol) that acts as both a killing agent and Overview and Detection preservative that holds the insects until they are collected. -

A Review of the Genus Amylostereum and Its Association with Woodwasps
70 South African Journal of Science 99, January/February 2003 Review Article A review of the genus Amylostereum and its association with woodwasps B. Slippers , T.A. Coutinho , B.D. Wingfield and M.J. Wingfield Amylostereum.5–7 Today A. chailletii, A. areolatum and A. laevigatum are known to be symbionts of a variety of woodwasp species.7–9 A fascinating symbiosis exists between the fungi, Amylostereum The relationship between Amylostereum species and wood- chailletii, A. areolatum and A. laevigatum, and various species of wasps is highly evolved and has been shown to be obligatory siricid woodwasps. These intrinsic symbioses and their importance species-specific.7–10 The principal advantage of the relationship to forestry have stimulated much research in the past. The fungi for the fungus is that it is spread and effectively inoculated into have, however, often been confused or misidentified. Similarly, the new wood, during wasp oviposition.11,12 In turn the fungus rots phylogenetic relationships of the Amylostereum species with each and dries the wood, providing a suitable environment, nutrients other, as well as with other Basidiomycetes, have long been unclear. and enzymes that are important for the survival and develop- Recent studies based on molecular data have given new insight ment of the insect larvae (Fig. 1).13–17 into the taxonomy and phylogeny of the genus Amylostereum. The burrowing activity of the siricid larvae and rotting of the Molecular sequence data show that A. areolatum is most distantly wood by Amylostereum species makes this insect–fungus symbio- related to other Amylostereum species. Among the three other sis potentially harmful to host trees, which include important known Amylostereum species, A. -

Technical Series, No
' ' Technical Series, No. 20, Part II. U. S. DEPARTMENT OF AGRICULTURE, BXJRE^TJ OK' TClSrTOM:OIL.OG^Y. L, 0. HOWARD, Entomologist and Chief of Bureau. TECHNICAL PAPERS ON MISCELLANEOUS .FOREST INSECTS. II. THE GENOTYPES OF THE SAWFLIES AND WOODWASPS, OR THE SUPERFAMILY TENTHKEDINOIDEA. S. A. ROHWER, Agent and Expert. Issued M.\rch 4, 1911. WASHINGTON: GOVERNMENT PRINTING OFFICE. 1911. Technical Series, No. 20, Part II. U. S. DEPARTMENT OF AGRICULTURE. L. 0. HOWARD, Entomologist and Chief of Bureau. TECHNICAL PAPERS ON MISCELLANEOUS FOREST INSECTS. II. THE GENOTYPES OF THE SAWFLIES AND WOODWASPS, OR THE SUPERFAMILY TENTHREDINOIDEA. BY S. A. ROHWER, Agent and Expert. Issued Makch 4, 1911. WASHINGTON: GOVERNMENT PRINTING OFFICE. 1911. B UREA U OF ENTOMOLOGY. L. O. Howard, Entomologist and Chief of Bureau. C. L. Marlatt, Entomologist and Acting Chief in Absence of Chief. R. S. Clifton, Executive Assistant. W. F. Tastet, Chief Clerk. F. H. Chittenden, in charge of truck crop and stored product insect investigations. A. D. Hopkins, in charge offorest insect investigations. W. D. Hunter, in charge of southern field crop insect investigations. F. M. Webster, in charge of cereal and forage insect investigations. A. L. Quaintance, in charge of deciduous fruit insect investigations. E. F. Phillips, in charge of bee culture. D. M. Rogers, in charge of preventing spread of moths, field -work. RoLLA P. Currie, in charge of editorial work. Mabel Colcord, librarian. , Forest Insect Investigations. A. D. Hopkins, in charge. H. E. Burke, J. L. Webb, Josef Brunner, S. A. Rohwer, T. E. Snyder, W. D. Edmonston, W. B. Turner, agents and experts. -

Commodity Risk Assessment of Black Pine (Pinus Thunbergii Parl.) Bonsai from Japan
SCIENTIFIC OPINION ADOPTED: 28 March 2019 doi: 10.2903/j.efsa.2019.5667 Commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan EFSA Panel on Plant Health (EFSA PLH Panel), Claude Bragard, Katharina Dehnen-Schmutz, Francesco Di Serio, Paolo Gonthier, Marie-Agnes Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas-Cortes, Stephen Parnell, Philippe Lucien Reignault, Hans-Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen, Lucia Zappala, Andrea Battisti, Anna Maria Vettraino, Renata Leuschner, Olaf Mosbach-Schulz, Maria Chiara Rosace and Roel Potting Abstract The EFSA Panel on Plant health was requested to deliver a scientific opinion on how far the existing requirements for the bonsai pine species subject to derogation in Commission Decision 2002/887/EC would cover all plant health risks from black pine (Pinus thunbergii Parl.) bonsai (the commodity defined in the EU legislation as naturally or artificially dwarfed plants) imported from Japan, taking into account the available scientific information, including the technical information provided by Japan. The relevance of an EU-regulated pest for this opinion was based on: (a) evidence of the presence of the pest in Japan; (b) evidence that P. thunbergii is a host of the pest and (c) evidence that the pest can be associated with the commodity. Sixteen pests that fulfilled all three criteria were selected for further evaluation. The relevance of other pests present in Japan (not regulated in the EU) for this opinion was based on (i) evidence of the absence of the pest in the EU; (ii) evidence that P. -

Pathways Analysis of Invasive Plants and Insects in the Northwest Territories
PATHWAYS ANALYSIS OF INVASIVE PLANTS AND INSECTS IN THE NORTHWEST TERRITORIES Project PM 005529 NatureServe Canada K.W. Neatby Bldg 906 Carling Ave., Ottawa, ON, K1A 0C6 Prepared by Eric Snyder and Marilyn Anions NatureServe Canada for The Department of Environment and Natural Resources. Wildlife Division, Government of the Northwest Territories March 31, 2008 Citation: Snyder, E. and Anions, M. 2008. Pathways Analysis of Invasive Plants and Insects in the Northwest Territories. Report for the Department of Environment and Natural Resources, Wildlife Division, Government of the Northwest Territories. Project No: PM 005529 28 pages, 5 Appendices. Pathways Analysis of Invasive Plants and Insects in the Northwest Territories i NatureServe Canada Acknowledgements NatureServe Canada and the Government of the Northwest Territories, Department of Environment and Natural Resources, would like to acknowledge the contributions of all those who supplied information during the production of this document. Canada : Eric Allen (Canadian Forest Service), Lorna Allen (Alberta Natural Heritage Information Centre, Alberta Community Development, Parks & Protected Areas Division), Bruce Bennett (Yukon Department of Environment), Rhonda Batchelor (Northwest Territories, Transportation), Cristine Bayly (Ecology North listserve), Terri-Ann Bugg (Northwest Territories, Transportation), Doug Campbell (Saskatchewan Conservation Data Centre), Suzanne Carrière (Northwest Territories, Environment & Natural Resources), Bill Carpenter (Moraine Point Lodge, Northwest -

SIREX NOCTILIO HOST CHOICE and NO-CHOICE BIOASSAYS: WOODWASP PREFERENCES for SOUTHEASTERN U.S. PINES by JAMIE ELLEN DINKINS (Und
SIREX NOCTILIO HOST CHOICE AND NO-CHOICE BIOASSAYS: WOODWASP PREFERENCES FOR SOUTHEASTERN U.S. PINES by JAMIE ELLEN DINKINS (Under the Direction of Kamal J.K. Gandhi) ABSTRACT Sirex noctilio F., the European woodwasp, is an exotic invasive pest newly introduced to the northeastern U.S. This woodwasp kills trees in the Pinus genus and could potentially cause millions of dollars of damage in the southeastern U.S., where pine plantations are extensive. At present, little is known about the preferences of this wasp for southeastern pine species, and further, little methodology exists as related to conducting host choice or no-choice bioassays with this species. My thesis developed methodology to successfully perform S. noctilio host choice and no-choice bioassays (both colonization and emergence from bolts), examined S. noctilio behavioral and developmental responses to southeastern U.S. pine species using bolts, and investigated possible mechanisms to explain these behavioral responses. Results indicated larger bolts were preferred to smaller bolts by S. noctilio, and P. strobus and P. virginiana were preferred out of six southeastern species in host choice bioassays. KEYWORDS: choice and no-choice bioassay, southeastern pines, European woodwasp, host preference, mechanisms, Sirex noctilio, Pinus SIREX NOCTILIO HOST CHOICE AND NO-CHOICE BIOASSAYS: WOODWASP PREFERENCES FOR SOUTHEASTERN U.S. PINES by JAMIE ELLEN DINKINS B.S. The University of Tennessee at Chattanooga, 2009 A Thesis Submitted to the Graduate Faculty of the University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE Athens, GA 2011 © 2011 Jamie Ellen Dinkins All Rights Reserved SIREX NOCTILIO HOST CHOICE AND NO-CHOICE BIOASSAYS: WOODWASP PREFERENCES FOR SOUTHEASTERN U.S. -

Efficacy of Kamona Strain Deladenus Siricidicola Nematodes for Biological Control of Sirex Noctilio in North America and Hybridisation with Invasive Conspecifics
A peer-reviewed open-access journal NeoBiota 44: 39–55Efficacy (2019) of Kamona strainDeladenus siricidicola nematodes for biological... 39 doi: 10.3897/neobiota.44.30402 RESEARCH ARTICLE NeoBiota http://neobiota.pensoft.net Advancing research on alien species and biological invasions Efficacy of Kamona strain Deladenus siricidicola nematodes for biological control of Sirex noctilio in North America and hybridisation with invasive conspecifics Tonya D. Bittner1, Nathan Havill2, Isis A.L. Caetano1, Ann E. Hajek1 1 Department of Entomology, Cornell University, Ithaca, NY 14853-2601, USA 2 USDA Northern Research Station, 51 Mill Pond Rd, Hamden, CT 06514, USA Corresponding author: Tonya D. Bittner ([email protected]) Academic editor: W. Nentwig | Received 7 October 2018 | Accepted 19 December 2018 | Published 4 April 2019 Citation: Bittner TD, Havill N, Caetano IAL, Hajek AE (2019) Efficacy of Kamona strainDeladenus siricidicola nematodes for biological control of Sirex noctilio in North America and hybridisation with invasive conspecifics. NeoBiota 44: 39–55. https://doi.org/10.3897/neobiota.44.30402 Abstract Sirex noctilio is an invasive woodwasp that, along with its symbiotic fungus, has killed pine trees (Pinus spp.) in North America and in numerous countries in the Southern Hemisphere. We tested a biological control agent in North America that has successfully controlled S. noctilio in Oceania, South Africa, and South America. Deladenus siricidicola nematodes feed on the symbiotic white rot fungus Amylostereum areolatum and can switch to being parasitic on S. noctilio. When parasitic, the Kamona nematode strain can sterilise the eggs of S. noctilio females. However, in North America, a different strain of D. siricidicola (NA), presumably introduced along with the woodwasp, parasitises but does not sterilise S. -
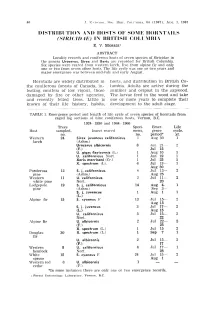
" ANI) HOSTS of SO"IE 1I0HNTAILS (SI Riel F)A F,,) in BH ITISII COLL'i BL\
00 .T . '·;'TO \lO I.. Se .... BIUT. CO I.I · \""A. 64 (1967)' A UG. 1. 1967 OISTHIBlTIO," ANI) HOSTS OF SO"IE 1I0HNTAILS (SI Riel f)A f,,) IN BH ITISII COLL'I BL\ E. V. MORRIS I ABSTRACT Locality recol'ds and con iferous hosts of seven species of Siricidae in the genera Urocerus, Sirex and Xeris are recorded for British Columbia. Six species were reared from western larch. fi ve from alpine fir and only one or two from seven other hosts. The life cycle was one or two years and major emergence was betw('('n mid-July ami early August. Horntails are widely dis tributed in hosts, and dis tribution in British Co the coniferous forests of Ca nada, in lumbia. Adults are active during the festing conifers of low vigour, those s ummer a nd ovipost in the sapwood. damaged by fire or other agencies, The la rvae fe ed in the wood and take and recently felled trees . Little is one or more years to complete their known of their life history, habits, development to the adult stage. TABLE 1. Emergence period and length of life cycle of sc' ven species of horn tails from caged log sections of nine coniferous hosts, Vernon, B.C. 1924 - 1930 and 1964 - 1966 Trees Speci Emer Life Host sampled, Insect reared mens, gence cycle, no. no. period* yr. Weste rn 24 Sirex iuvencus californicus 1 Aug 30 1 larch (Ashm. ) Urocerus albicornis 8 .Jun 21 ~ 2 (F.) Jul 15 U. gigas flavicornis (L.) 1 Aug 10 1 U. -

NEMATODES for the BIOLOGICAL CONTROL of the WOODWASP, SIREX NOCTILIO Robin A
NEMATODES FOR THE BIOLOGICAL CONTROL OF THE WOODWASP, SIREX NOCTILIO Robin A. Bedding CSIRO Entomology, PO Box 1700, ACT 2601, Australia ABSTRACT The tylenchid nematode Beddingia (Deladenus) been placed in a separate family of nematodes. siricidicola (Bedding) is by far the most important Poinar et al. [2002] have now placed both control agent of Sirex noctilio F., a major forms of Beddingia species in a new family, the pest of pine plantations. It sterilizes female Beddingiidae). sirex, is density dependent, can achieve nearly At about the time adult parasitized sirex emerge 100 percent parasitism and, as a result of its from infested trees, adult nematodes complicated biology can be readily manipulated have usually released most of the juveniles that are for sirex control. Bedding and Iede (2005) gave a within them into the insect’s blood cavity and the comprehensive account of this nematode and its juveniles have migrated to the insect’s reproductive use in Australia and South America. organs. In the male, this is a dead end but Throughout the 1960s and early 1970s, CSIRO and female sirex are effectively sterilized because, as various consultants conducted a comprehensive well as ovarial development being suppressed search, in hundreds of localities from Europe, to various degrees, each egg that is produced USA, Canada, India, Pakistan, Turkey, Morocco is fi lled with up to 200 juvenile nematodes. A and Japan, for coniferous trees infested with parasitized female sirex still oviposits readily, and any siricid species. Thousands of logs from these often in several different trees, but lays packets trees were then caged in quarantine, mainly at of nematodes instead of viable eggs.