Selected Birth Defects Data from Population-Based Birth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differential Diagnosis of Pulmonic Stenosis by Means of Intracardiac Phonocardiography
Differential Diagnosis of Pulmonic Stenosis by Means of Intracardiac Phonocardiography Tadashi KAMBE, M.D., Tadayuki KATO, M.D., Norio HIBI, M.D., Yoichi FUKUI, M.D., Takemi ARAKAWA, M.D., Kinya NISHIMURA,M.D., Hiroshi TATEMATSU,M.D., Arata MIWA, M.D., Hisao TADA, M.D., and Nobuo SAKAMOTO,M.D. SUMMARY The purpose of the present paper is to describe the origin of the systolic murmur in pulmonic stenosis and to discuss the diagnostic pos- sibilities of intracardiac phonocardiography. Right heart catheterization was carried out with the aid of a double- lumen A.E.L. phonocatheter on 48 pulmonic stenosis patients with or without associated heart lesions. The diagnosis was confirmed by heart catheterization and angiocardiography in all cases and in 38 of them, by surgical intervention. Simultaneous phonocardiograms were recorded with intracardiac pressure tracings. In valvular pulmonic stenosis, the maximum ejection systolic murmur was localized in the pulmonary artery above the pulmonic valve and well transmitted to both right and left pulmonary arteries, the superior vena cava, and right and left atria. The maximal intensity of the ejection systolic murmur in infundibular stenosis was found in the outflow tract of right ventricle. The contractility of the infundibulum greatly contributes to the formation of the ejection systolic murmur in the outflow tract of right ventricle. In tetralogy of Fallot, the major systolic murmur is caused by the pulmonic stenosis, whereas the high ventricular septal defect is not responsible for it. In pulmonary branch stenosis, the sys- tolic murmur was recorded distally to the site of stenosis. Intracardiac phonocardiography has proved useful for the dif- ferential diagnosis of various types of pulmonic stenosis. -

Tetralogy of Fallot: Stroke in a Young Patient
Open Access Case Report DOI: 10.7759/cureus.2714 Tetralogy of Fallot: Stroke in a Young Patient Hassam Ali 1 , Shiza Sarfraz 2 , Muhammad Sanan 1 1. Medical 4, Bahawal Victoria Hospital, Quaid-E-Azam Medical College, Bahawalpur, PAK 2. Department of Anesthesiology, Bahawal Victoria Hospital, Quaid-E-Azam Medical College, Bahawalpur, PAK Corresponding author: Hassam Ali, [email protected] Abstract Tetralogy of Fallot (TOF) is a congenital birth defect of the heart which actually comprises four individual flaws. It causes poor flow of oxygenated blood to the organs and leads to cyanosis (blue-tinted skin, because of inadequate oxygenation). It can be recognized at birth or in adulthood. But sometimes, cases may go unnoticed, and the patient might present with some rare complications. In this case, the patient presented with an embolic infarct of the brain at the age of 25 with an undiagnosed tetralogy of Fallot. Categories: Cardiology, Internal Medicine, Neurology Keywords: congenital heart defects, embolic stroke, tetralogy of fallot, paralysis, cardiology, cerebrovascular accident, heart, neurology, international medicine Introduction Tetralogy of Fallot (TOF) is a heart disease present at birth [1]. It consists of four defects [2]: - Pulmonary stenosis - Ventricular septal defect - Right ventricular hypertrophy - Aorta overriding the ventricular septal defect Some babies, when they cry or breastfeed, may turn very blue. This is because of "a tet spell" due to the shunting of excess deoxygenated blood from the right chamber of the heart to the left chamber. Such babies may have difficulty breathing, become limp, or even lose consciousness [3]. TOF is treated by open-heart surgery, mostly in the first year of life. -

Trilogy of Fallot in a Dog
J Vet Clin 29(5) : 404-407 (2012) Trilogy of Fallot in a Dog Ran Choi, Hyo-Jin Ahn and Changbaig Hyun1 Section of Small Animal Internal Medicine, College of Veterinary Medicine, Kangwon National University, Chuncheon 201-100, Korea (Accepted: October 05, 2012) Abstract : A 3 years-old female mixed dog (weighing 5.3 kg) was referred to veterinary teaching hospital of Kangwon National University with primary complaints of syncope, severe exercise intolerance, depression and lethargy. Diagnostic studies revealed polycythemia, right sided cardiac enlargement on thoracic radiography and right-to left atrial septal defect, severe pulmonary stenosis (~5 m/s of peak velocity) and right ventricular hypertrophy. Based on diagnostic findings, the dog was diagnosed as trilogy of Fallot. To improve clinical condition of this dog, diltiazem and enalapril were prescribed with weekly phlebotomy. To author’s best knowledge, this is the first case of trilogy of Fallot in Korea. Key words : trilogy of Fallot, atrial septal defects, pulmonic stenosis, right ventricular hypertrophy, CHD. Introduction ring veterinarian, the dog had history of cardiac murmur after birth and occasional syncopal episodes (increasing frequency Trilogy of Fallot is a compound congenital heart defects recently). On the physical examination, the dog had grade 2/6 (CHD) comprised of pulmonary stenosis, atrial septal dys- mild systolic murmur at right apex and left base of heart. The morphogenesis (e.g. atrial septal defect and patent foramen systolic blood pressure measured by Doppler method was ovale) and right ventricular hypertrophy (1). The CHD are 100-110 mmHg. broadly classified into cyanotic CHD and non-cyanotic CHD. -
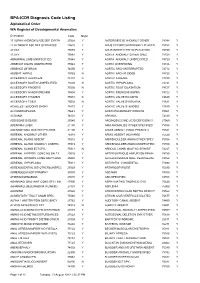
BPA-ICD9 Diagnosis Code Listing Alphabetical Order WA Register of Developmental Anomalies
BPA-ICD9 Diagnosis Code Listing Alphabetical Order WA Register of Developmental Anomalies Description Code Major 17 ALPHA HYDROXYLASE DEF SYNTH25524 Y ANTERIOR EYE ANOMALY OTHER74348 Y 21 HYDROXYLASE DEF SYNTHESIS25520 Y ANUS ECTOPIC/ANTERIORLY PLACED75153 Y 47,XXX 75885 Y ANUS/INTEST/CYST DUPLICATION75150 Y 47,XYY 75884 Y AORTA ANOMALY OTHER SPEC74728 Y ABNORMAL LIMB UNSPECIFIED75548 Y AORTA ANOMALY UNSPECIFIED74729 Y ABSENCE DIGITS UNSPECIFIED75544 Y AORTA OVERRIDING 74726 Y ABSENCE OF BRAIN 74000 Y AORTIC ARCH INTERRUPTED 74712 Y ABSENT NIPPLE 75763 N AORTIC ARCH R SIDED 74723 Y ACCESSORY AURICLE/S 74410 N AORTIC ATRESIA 74720 Y ACCESSORY DIGIT/S UNSPECIFIED75509 N AORTIC HYPOPLASIA 74721 Y ACCESSORY FINGER/S 75500 N AORTIC ROOT DILATATION 74727 Y ACCESSORY HAND/FOREARM 75504 Y AORTIC STENOSIS SUPRA 74722 Y ACCESSORY THUMB/S 75501 N AORTIC VALVE BICUSPID 74640 Y ACCESSORY TOE/S 75502 N AORTIC VALVE DYSPLASIA 74631 Y ACHILLES TENDON/S SHORT 75472 Y AORTIC VALVE STENOSIS 74630 Y ACHONDROPLASIA 75643 Y AORTOPULMONARY WINDOW 74501 Y ACRANIA 74001 Y APHAKIA 74330 Y ADDISONS DISEASE 25540 Y ARGINOSUCCINIC ACID DEFICIENCY27060 Y ADENOMA LIVER 21150 Y ARM ANOMALIES OTHER SPECIFIED75556 Y ADENOMYOMA GASTRIC PYLORIS21100 Y ARM/S ABSENT (HAND PRESENT)75521 Y ADRENAL ANOMALY OTHER 75918 Y ARM/S ABSENT (NO HAND) 75520 Y ADRENAL GLAND ABSENT 75910 Y ARM/SHOULDER ANOM OTHER SPEC75558 Y ADRENAL GLAND ANOMALY UNSPEC75919 Y ARM/SHOULDER ANOM UNSPECIFIED75559 N ADRENAL GLAND ECTOPIC 75913 N ARNOLD CHIARI MALF NO SPIN BIF74227 Y ADRENAL HYPERPL CONG NO -
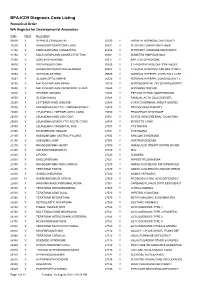
BPA-ICD9 Diagnosis Code Listing Numerical Order WA Register for Developmental Anomalies
BPA-ICD9 Diagnosis Code Listing Numerical Order WA Register for Developmental Anomalies Code Major Description 09090 Y SYPHILIS CONGENITAL 25330 Y GROWTH HORMONE DEFICIENCY 16200 Y RHABDOMYOSARCOMA LUNG 25331 Y PITUITARY DWARFISM OTHER 17100 Y FIBROSARCOMA CONGENITAL 25342 Y PITUITARY HORMONE DEFICIENCY 17190 Y MALIG NEOPLASM CONNECTIVE TISS 25351 Y DIABETES INSIPIDUS NOS 17300 Y GORLIN SYNDROME 25511 Y BARTTER SYNDROME 18690 Y ORCHIOBLASTOMA 25520 Y 21 HYDROXYLASE DEF SYNTHESIS 18900 Y RHABDOMYOSARCOMA BLADDER 25524 Y 17 ALPHA HYDROXYLASE DEF SYNTH 19050 Y RETINOBLASTOMA 25525 Y ADRENAL HYPERPL CONG SALT LOSS 19051 Y GLIOMA OPTIC NERVE 25526 Y ADRENAL HYPERPL CONG NO SALT L 19100 Y MALIG NEOPLASM BRAIN 25529 Y ADRENOGENTIAL DIS OTHER&UNSPEC 19400 Y MALIG NEOPLASM ENDOCRINE GLAND 25540 Y ADDISONS DISEASE 19410 Y NEUROBLASTOMA 25543 Y PSEUDO HYPOALDOSTERONISM 19500 Y GLIOMA NASAL 25548 Y FAMILIAL ACTH (GLUCOID DEF) 20250 Y LETTERER-SIWE DISEASE 25549 Y CORTICOADRENAL INSUFF UNSPEC 20290 Y HAEMOPHAGOCYTIC LYMPHOHISTIOCY 25910 Y PRECOCIOUS PUBERTY 20400 Y LEUKAEMIA LYMPHOBLASTIC CONG 25980 Y PROGEROID SYNDROME 20500 Y LEUKAEMIA MYELOID CONG 25981 Y SOTOS SYN/CEREBRAL GIGANTISM 20600 Y LEUKAEMIA MONOCYTIC ACUTE CONG 26800 Y RICKETTS CONG 20890 Y LEUKAEMIA CONGENITAL NOS 27000 Y CYSTINOSIS 21000 Y MYOFIBROMA TONGUE 27001 Y CYSTINURIA 21100 Y ADENOMYOMA GASTRIC PYLORIS 27002 Y FANCONI SYNDROME 21150 Y ADENOMA LIVER 27003 Y HARTNUP DISEASE 21270 Y RHABDOMYOMA HEART 27009 Y AMINO-ACID TRNSPT DISTRB OTHER 21300 Y OSTEOCHONDROMATA 27010 Y PKU 21400 -

The Clinical Evolution of Shunt-Operations for Morbus Caeruleus: Results of 150 Operations, in a Long-Term Follow-Up
The Clinical Evolution of Shunt-Operations for Morbus Caeruleus: Results of 150 Operations, in a Long-Term Follow-Up C. A. Mahuim, M.D., C.L.C. van Nieuwenhuizen, M.D., H.A.H. D’heer, M.D., and F. Sloojj, M.D., Utrecht, Netherlands INTRODUCTION The classical operations of Blalock,4 Potts,25 and Brocks which have been used for more than 10 years in treating the tetralogy of Fallot are purely symp- tomatic. Their aim is to augment the pulmonary circulation in order to increase the oxygenation of the blood. However, they do not, properly speaking, correct the malformation. In fact, with the procedures of Blalock and Potts a fifth malformation is created in a heart that already has four. It is well known that an operation which creates an artificial ductus arteriosus must, in the long run, produce a significant overloading of the heart. In any event, the immediate benefits of the operation, in so far as the general status of the patients and the amelioration of the cyanosis are concerned, are so evident that the undeniable usefulness of this type of operation is universally recognized. TABLE I GOOD AUTHOR AND NUMBER PROCEDURE USED (IN OVER-ALL I UZSULTS YEAR OF ORDEROFFREQUENCY) MORTALITY (PER FOLLOW-UP PATIENTS (PER CENT) CENT) -- Potts, 1956 514 Potts 9 ? ? Johnson, 1951 144 Blalock, Potts 12 ? ? Bret, 1952 172 Potts, Blalock, Brock 20 i Derra, 1952 242 Blalock. Brock 19 7’: ? Dubost, 1954 333 Blalock; Potts, Brock 16 78 ? Brock, 1955 140 Brock 11.5 ? Sellers, 1950 Blalock, Potts, Brock 10.5 E.5 6 mo. -

Cardiac Surgery Was Performed Under Beating Heart
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Review REVIEW On-pump Beating Heart Surgery Ansheng Mo, MD a,∗ and Hui Lin, MD a,b a Department of Cardiothoracic Surgery, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning 530021, China b The People’s Hospital of Wuhan University, Wuhan, China On-pump beating heart surgery has been proposed as a superior technique for cardiac surgery, and is receiving renewed interest from cardiac surgeons. Here, we review the technique, focusing on the basic principles of myocardial protection, operative methodology, category, intraoperative concerns, current status, organ protection, advantages, disadvantages, indications, contraindications, unanswered questions, and future research. (Heart, Lung and Circulation 2011;20:295–304) © 2011 Australasian Society of Cardiac and Thoracic Surgeons and the Cardiac Society of Australia and New Zealand. Published by Elsevier Inc. All rights reserved. Keywords. On-pump; Beating heart; Ischaemia–reperfusion Introduction Feasibility of Surgery on the Empty Beating Heart ardiac surgery was performed under beating heart Myocardial Protection C or ventricular fibrillation conditions prior to the During on-pump beating heart surgery, the oxygen advent of cardioplegia, which made surgery easier. -

A Retrospective Study of Tetralogy of Fallot in Dogs
Turkish Journal of Veterinary and Animal Sciences Turk J Vet Anim Sci (2013) 37: 57-60 http://journals.tubitak.gov.tr/veterinary/ © TÜBİTAK Research Article doi:10.3906/vet-1108-30 A retrospective study of tetralogy of Fallot in dogs 1 1 1, 1 2 Urszula PASŁAWSKA , Agnieszka NOSZCZYK-NOWAK , Alicja CEPIEL *, Maciej STASZCZYK , Adrian JANISZEWSKI 1 Department of Internal Medicine Clinic of Diseases of Horses, Dogs, and Cats, Faculty of Veterinary Medicine, Wroclaw University of Environmental and Life Sciences, Wroclaw, Poland 2 Regional Specialist Hospital in Wroclaw, Research and Development Center, Wroclaw, Poland Received: 30.08.2011 Accepted: 04.01.2012 Published Online: 22.01.2013 Printed: 22.02.2013 Abstract: Tetralogy of Fallot is a rare congenital heart defect; however, it is the most common cyanotic heart malformation in humans and animals. It consists of a ventricular septal defect, pulmonic stenosis, displacement of the aortic root, and hypertrophy of the right ventricle. At the Department of Internal Medicine Clinic of Diseases of Horses, Dogs, and Cats at the Wroclaw University of Environmental and Life Sciences, patients with Fallot syndrome constituted 4.44% of all patients with congenital heart diseases. There was no breed predisposition found. The most common clinical signs were exercise intolerance, dyspnea, and cyanosis of the mucous membranes, but it can also be asymptomatic. Echocardiography is considered to be the best method for diagnosing this congenital heart disease. Contrast angiocardiography can be used to confirm the diagnosis or as a complement to diagnosis. Surgery is the only effective method of treatment; however, for animals, we only provide palliative treatment. -

Longevity in the Tetralogy of Fallot H
Thorax: first published as 10.1136/thx.19.1.12 on 1 January 1964. Downloaded from Tlhorax (1964), 19, 12 Longevity in the tetralogy of Fallot H. MEINDOK From University College Hospital, London The congenital abnormality of the heart consisting As a schoolboy he had loved cricket and played of pulmonary stenosis, ventricular septal defect, sometimes two games of football a day. At the age and an overriding aorta was described in 1673 of 10 he was told at King's College Hospital, London, by the Danish scholar, Nicolas Stensen (Warburg, that he had 'heart trouble'. He was rejected by the 1942). Fallot's (Fallot, 1888) description included army medical board when he tried to volunteer in 1915, because of his 'heart'. right ventricular hypertrophy as an essential part For the last 27 years he had worked as a fettler, of the malformation. Stensen had made no a moderately heavy job consisting of trimming and mention of this, possibly because he reported on lifting heavy metal castings in a foundry. He was the heart of the foetus. married, had one daughter, was a moderate beer Maud Abbott (1936) found in her series of 1,000 drinker, and smoked 10 cigarettes a day. cases of congenital heart disease that the average He had been very well until the summer of 1955, life expectancy in Fallot's tetralogy was 12 years, when he noticed shortness of breath on walking up and only six years in cases with pulmonary artery a hill on his way to work. atresia. In one report on 200 living patients On admission in 1957, there was no dyspnoea at rest, no clubbing, but central cyanosis. -

Cyanotic Heart Disease Beyond Neonatal Period Tetralogy of Fallot Components
William Herring, M.D. © 2003 CyanoticCyanotic HeartHeart DiseaseDisease In Slide Show mode, to advance slides, press spacebar or click left mouse button Cyanosis With Decreased Vascularity O Tetralogy O Truncus-type IV O Tricuspid atresia* O Transposition* O Ebstein's * Also appears on DDx of Cyanosis with ↑ Vascularity Cyanosis With Increased Vascularity O Truncus types I, II, III O TAPVR O Tricuspid atresia* O Transposition* O Single ventricle * Also appears on DDx of Cyanosis with ↓ Vascularity TetralogyTetralogy ofof FallotFallot Tetralogy of Fallot General • About 10% of all congenital heart lesions • Most common cause of cyanotic heart disease beyond neonatal period Tetralogy of Fallot Components • High VSD • Pulmonic stenosis, i.e. right ventricular outflow obstruction • Usually infundibular, sometimes valvular • Overriding of the aorta • Right ventricular hypertrophy 1 2 3 4 © Frank Netter, MD Novartis® Tetralogy of Fallot Tetralogy of Fallot Other anomalies • Right aortic arch in 25% • Mirror image type • Left superior vena cava • ASD • Tricuspid valve abnormalities • Anomalies of coronary arteries • Aberrant left anterior descending coronary artery arising from right coronary artery Tetralogy of Fallot Other anomalies • Abnormalities of the pulmonary artery and its branches • Peripheral PA coarctations, unilateral • Absence or hypoplasia of pulmonary artery • Usually left • Absence of pulmonic valve • Bicuspid pulmonic valve Tetralogy of Fallot Critical Component • Degree of pulmonic stenosis • Regulates degree of R « L shunt -

Dual-Source Computed Tomography Evaluation of Children with Congenital Pulmonary Valve Stenosis
Iran J Radiol. 2016 April; 13(2):e34399. doi: 10.5812/iranjradiol.34399. Published online 2016 March 1. Research Article Dual-Source Computed Tomography Evaluation of Children with Congenital Pulmonary Valve Stenosis Zhanguo Sun,1,2 Wenjian Xu,1,* Shuran Huang,2 Yueqin Chen,2 Xiang Guo,2 and Zhitao Shi2 1Department of Radiology, Affiliated Hospital of Qingdao University, Qingdao City, China 2Shandong Provincial Key Laboratory of Cardiac Disease Diagnosis and Treatment, Affiliated Hospital of Jining Medical University, Jining, China *Corresponding author: Wenjian Xu, Department of Radiology, Affiliated Hospital of Qingdao University, Qingdao City, Shandong, 266071, China, E-mail: [email protected] Received 2015 November 05; Revised 2015 December 22; Accepted 2016 January 05. Abstract Background: Despite dual-source computed tomography (DSCT) technology has been performed well on adults or infants with heart disease, specific knowledge about children with congenital pulmonary valve stenosis (PS) remained to be established. Objectives: This original research aimed to establish a professional approach of DSCT performing technology on children and to assess the image quality performed by DSCT to establish a diagnostic evaluation for children with PS. Patients and Methods: Ninety-eight children with congenital PS referred to affiliated hospital of Jining medical college were re- cruited from October 2013 to March 2015. Participants were divided into four groups according to different ages (0 - 1, 1 - 3, 3 - 7, 7 - 14), or three groups according to different heart rates (< 90, 90 - 110, > 110). Image quality of pulmonary valves was assessed based on a four-point grading scale (1 - 4 points). Those cases achieving a score of ≥ 3 points were selected for further investigation, which played a critical role in our analysis. -

Appendix L Manual 2016-2017 V1.1
APPENDIX L Validation Messages Explained Queensland Hospital Admitted Patient Data Collection QHAPDC 2016-2017 V1.1 Appendix L Published by the State of Queensland (Queensland Health), January 2017 This document is licensed under a Creative Commons Attribution 3.0 Australia licence. To view a copy of this licence, visit creativecommons.org/licenses/by/3.0/au © State of Queensland (Queensland Health) 2017 You are free to copy, communicate and adapt the work, as long as you attribute the State of Queensland (Queensland Health). For more information contact: Statistical Collections and Integration Unit, Statistical Services Branch, Strategy, Policy and Planning Division, Department of Health, GPO Box 48, Brisbane QLD 4001, email [email protected], phone 07 3224 8741. An electronic version of this document is available at https://www.health.qld.gov.au/hsu/collections/qhapdc.asp Disclaimer: The content presented in this publication is distributed by the Queensland Government as an information source only. The State of Queensland makes no statements, representations or warranties about the accuracy, completeness or reliability of any information contained in this publication. The State of Queensland disclaims all responsibility and all liability (including without limitation for liability in negligence) for all expenses, losses, damages and costs you might incur as a result of the information being inaccurate or incomplete in any way, and for any reason reliance was placed on such information. APPENDIX L – 2016-2017 v1.1 - 2 - Contents