The Isolation of Secondary Metabolites from Rheum Ribes L. and The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Azerbaijan Azerbaijan
COUNTRY REPORT ON THE STATE OF PLANT GENETIC RESOURCES FOR FOOD AND AGRICULTURE AZERBAIJAN AZERBAIJAN National Report on the State of Plant Genetic Resources for Food and Agriculture in Azerbaijan Baku – December 2006 2 Note by FAO This Country Report has been prepared by the national authorities in the context of the preparatory process for the Second Report on the State of World’s Plant Genetic Resources for Food and Agriculture. The Report is being made available by the Food and Agriculture Organization of the United Nations (FAO) as requested by the Commission on Genetic Resources for Food and Agriculture. However, the report is solely the responsibility of the national authorities. The information in this report has not been verified by FAO, and the opinions expressed do not necessarily represent the views or policy of FAO. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of FAO concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views of FAO. CONTENTS LIST OF ACRONYMS AND ABBREVIATIONS 7 INTRODUCTION 8 1. -
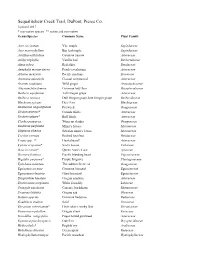
Sequalitchew Creek Trail Plant List
Sequalitchew Creek Trail, DuPont, Pierce Co. Updated 2017 * non-native species ** native and non-native Genus/Species Common Name Plant Family Acer circinatum Vine maple Sapindaceae Acer macrophyllum Big leaf maple Sapindaceae Achillea millefolium Common yarrow Asteraceae Achlys triphylla Vanilla leaf Berberidaceae Alnus rubra Red alder Betulaceae Anaphalis margaritacea Pearly everlasting Asteraceae Arbutus menziesii Pacific madrone Ericaceae Artemisia suksdorfii Coastal wormwood Asteraceae Asarum caudatum Wild ginger Aristolochiaceae Athyrium felix-femina Common lady fern Dryopteridaceae Berberis aquifolium Tall Oregon grape Asteraceae Berberis nervosa Dull Oregon grape, low Oregon grape Berberidaceae Blechnum spicant Deer fern Blechnaceae Chamerion angustifolium Fireweed Onagraceae Cirsium arvense* Canada thistle Asteraceae Cirsium vulgare* Bull thistle Asteraceae Clarkia purpurea Winecup clarkia Onagraceae Claytonia perfoliata Miner's lettuce Montiaceae Claytonia siberica Siberian miner's lettuce Montiaceae Corylus cornuta Beaked hazelnut Betulaceae Crepis spp. ?* Hawksbeard? Asteraceae Cytisus scoparius* Scot's broom Fabaceae Daucus carota* Queen Anne's Lace Apiaceae Dicentra formosa Pacific bleeding heart Papaveraceae Digitalis purpurea* Purple foxglove Plantaginaceae Epilobium minutum Threadstem fireweed Onagraceae Equisetum arvense Common horsetail Equisetaceae Equisetum telmateia Giant horsetail Equisetaceae Eriophyllum lanatum Oregon sunshine Asteraceae Erythronium oregonum White fawn lily Liliaceae Frangula purshiana Cascara, -

City of Vancouver Native Trees and Shrubs Last Revision: 2010 Plant Characteristics (A - M)
City of Vancouver Native Trees and Shrubs Last Revision: 2010 Plant Characteristics (A - M) *This list is representative, but not exhaustive, of the native trees and shrubs historically found in the natural terrestrial habitats of Vancouver, Washington. Botanical Name Common NameGrowth Mature Mature Growth Light / Shade Tolerance Moisture Tolerance Leaf Type Form Height Spread Rate Full Part Full Seasonally Perennially Dry Moist (feet) (feet) Sun Sun Shade Wet Wet Abies grandies grand fir tree 150 40 medium evergreen, 99 999 conifer Acer circinatum vine maple arborescent 25 20 medium deciduous, shrub 99 99 broadleaf Acer macrophyllum bigleaf maple tree 75 60 fast deciduous, 99 999 broadleaf Alnus rubra red alder tree 80 35 very fast deciduous, 99 999 broadleaf Amalanchier alnifolia serviceberry / saskatoon arborescent 15 8 medium deciduous, shrub 99 99 broadleaf Arbutus menziesii Pacific madrone tree 50 50 very slow evergreen, 99 9 broadleaf Arctostaphylos uva-ursi kinnikinnick low creeping 0.5 mat- fast evergreen, shrub forming 999 broadleaf Berberis aquifolium tall Oregon-grape shrub 8 3 medium evergreen, (Mahonia aquilfolium) 99 99 broadleaf Berberis nervosa low Oregon-grape low shrub 2 3 medium evergreen, (Mahonia aquifolium) 99 9 99 broadleaf Cornus nuttalli Pacific flowering dogwood tree 40 20 medium deciduous, 99 99 broadleaf Cornus sericea red-osier dogwood shrub 15 thicket- very fast deciduous, forming 99 9 9 9 broadleaf Corylus cornuta var. californica California hazel / beaked shrub 20 15 fast deciduous, hazelnut 99 9 9 broadleaf -

Chemical Constituents of Rheum Ribes L
Available online on www.ijppr.com International Journal of Pharmacognosy and Phytochemical Research 2017; 9(1); 65-69 DOI number: 10.25258/ijpapr.v9i1.8042 ISSN: 0975-4873 Research Article Chemical Constituents of Rheum ribes L. Consolacion Y Ragasa1,2,*, Jariel Naomi B Bacar1, Maria Margarita R Querido1, Maria Carmen S Tan1, Glenn G Oyong3, Robert Brkljača4, Sylvia Urban4 1Chemistry Department, De La Salle University, 2401 Taft Avenue, Manila 1004, Philippines 2Chemistry Department, De La Salle University Science & Technology Complex Leandro V. Locsin Campus, Biñan City, Laguna 4024, Philippines 3Biology Department, De La Salle University, 2401 Taft Avenue, Manila 1004, Philippines 4School of Science (Discipline of Applied Chemistry and Environmental Science), RMIT University (City Campus), Melbourne 3001, Victoria, Australia Received: 10th Sept, 16; Revised: 12th Dec, 16; Accepted: 20th Dec,16; Available Online: 15th January, 2017 ABSTRACT Chemical investigation of the dichloromethane extract of Rheum ribes has led to the isolation of β-sitosteryl-3β- glucopyranoside-6'-O-fatty acid esters (1), β-sitosterol (2), phytyl fatty acid esters (3), triacylgly c e r o l s (4) and chlorophyllide a (5). The structures of 1-5 were identified by comparison of their NMR data with literature data. Keywords: Rheum ribes L., Polygonaceae, β-sitosteryl-3β-glucopyranoside-6'-O-fatty acid esters, β-sitosterol, phytyl fatty acid esters, triacylglycerols, chlorophyllide a INTRODUCTION acid (3.64%). The essential oil was also evaluated for Rheum ribes L. of the family Polygonaceae, locally known general toxicity using a bioassay brine shrimp lethality as “Rivas” is a native plant of Iran which grows in several method. -

Genetic Characterization of Rheum Ribes (Wild Rhubarb) Genotypes in Lake Van Basin of Turkey Through ISSR and SSR Markers
INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY ISSN Print: 1560–8530; ISSN Online: 1814–9596 18–1295/2019/21–4–795–802 DOI: 10.17957/IJAB/15.0958 http://www.fspublishers.org Full Length Article Genetic Characterization of Rheum ribes (Wild Rhubarb) Genotypes in Lake Van Basin of Turkey through ISSR and SSR Markers Aytekin Ekincialp1*, Ceknas Erdinc2, Sibel Turan2, Ozlem Cakmakci3, Muhammad Azhar Nadeem4, Faheem Shehzad Baloch4 and Suat Sensoy3 1Van Yuzuncu Yil University, Baskale Vocational School, Van, Turkey 2Van Yuzuncu Yil University, Faculty of Agriculture, Agricultural Biotechnology Department, Van, Turkey 3Van Yuzuncu Yil University, Faculty of Agriculture, Horticulture Department, Van, Turkey 4Department of Field Crops, Faculty of Agricultural and Natural Science, Abant Izzet Baysal University, Bolu, Turkey *For correspondence: [email protected] Abstract Rheum ribes L. (wild rhubarb) is one of the less known plants to the world and the only species from the Rheum genus present in Turkey. In this study, one R. rhabarbarum (as check genotype) and 80 R. ribes genotypes were collected from different geographical locations of Turkey for the investigation of diversity and genetic structure using ISSR (Inter Simple Sequence Repeat) and SSR (Simple Sequence Repeats) markers. SSR markers reflected higher (100%) polymorphism as compared to the ISSR marker. However, ISSR markers produced higher average Polymorphism Information Content (PIC) value (0.805) than the SSR markers (0.724). A Similar range of (PIC) values with ISSR markers was found greater (0.935-0.395) as compared to the range of SSR makers (0.88-0.47). Using Jaccard similarity index, genetic distance was measured for both markers and average genetic distance was found to be higher with ISSR markers as compared to the SSR markers. -

In Vitro Evaluation of Rheum Ribes Induced Genotoxicity in Hepg2 Cell Lines
Istanbul J Pharm 49 (3): 132-136 DOI: 10.26650/IstanbulJPharm.2019.19021 Original Article In vitro evaluation of Rheum ribes induced genotoxicity in HepG2 cell lines Mahmoud Abudayyak Department of Pharmaceutical Toxicology, Karadeniz Technical University, Faculty of Pharmacy, Trabzon, Turkey ORCID ID of the author: M.A. 0000-0003-2286-4777. Cite this article as: Abudayyak M (2019). In vitro evaluation of Rheum ribes induced genotoxicity in HepG2 cell lines. Istanbul J Pharm 49 (3): 132-136. ABSTRACT Rheum ribes is a perennial herbaceous plant belonging to the Polygonaceae family that grows more on rocky and gravelly slopes in high altitude areas of Levant and Turkey. Rheum ribes is consumed as food and widely used in folk medicine against nausea, constipation and for different diseases including diabetes and hypertension. Unfortunately, the research on Rheum ribes toxicity is insufficient. In our study, the human hepatocellular carcinoma (HepG2) cell line was used in a cytotoxicity evaluation of Rheum ribes water, methanol and chloroform extracts by MTT and NRU tests. Comet assay was used to inves- tigate the genotoxicity potentials of the plant extracts. Our results show that all extracts cause cell death in a concentration dependent manner at 5-50 mg/mL concentrations. The IC50 values are 14.29-31.94 mg/mL by MTT and 21.15-27.66 mg/mL by NRU assay. The highest concentration (25 mg/mL) of methanol extract causes significant DNA damage (8.7-folds). In conclu- sion, similar to a lot of plants used in folk medicine the risk of Rheum ribes is still unknown. -

Effect of Hot Aqueous Extract of Rheum Ribes Roots on Some Hormonal and Biochemical Parameters in Induced Polycystic Ovary Syndrome in Local Female Rabbits
EurAsian Journal of BioSciences Eurasia J Biosci 12, 419-423 (2018) Effect of hot aqueous extract of Rheum ribes roots on some hormonal and biochemical parameters in induced Polycystic Ovary Syndrome in local female rabbits Aseel Mokdad Hatam AbdulWahed 1, Salih M. Rahem Al-obaidi 2, Abdul-monaim H. M. Al-samarrai 3 1 Samarra university, College of applied sciences, Department of analysis pathological, IRAQ 2 Tikrit university, college of education and pure sciences, department of biology, IRAQ 3 Samarra university, College of education, Department of chemistry, IRAQ Abstract The study aimed to evaluate the effect of aqueous extract of R. ribes roots and metformin in treatment of induced PCOS. 40 adult female rabbits (1000-1600g) use in this study, 30 rabbits injected with TP (100mg/Kg) for 4 days consecutive and left 3 days for developing the syndrome. The female rabbits divided to four groups: C+ve group, C-ve group, G1 (R. ribes extract 300mg/Kg) and G2 (metformin group 20mg/Kg). Treatment period was 30 days. The hormonal and biochemical assays include: AMH, Testosterone, Insulin, HOMA-IR and Glucose levels. The results showed significant increase at P≥0.05 in levels of AMH, Testosterone, Insulin, HOMA-IR and Glucose in C+ve group compared to C-ve group while the treated groups (G1 and G2) showed significant decrease in a parameters compared with C+ve except Insulin levels in G2 which show non-significant difference compared to C-ve, C+ve and G1. Keywords: PCOS, AMH, HOMA-IR, R. ribes AbdulWahed AMH, Al-obaidi SMR, Al-samarrai A-MHM (2018) Effect of hot aqueous extract of Rheum ribes roots on some hormonal and biochemical parameters in induced Polycystic Ovary Syndrome in local female rabbits. -

Plot B510 Plot D305 Close-Up and Overview Photos of PEM1
Plot A641 Plot B510 Plot C308 Plot D305 Close-up and overview photos of PEM1 Vegetation Community during June 2010 Plot C341 Plot D530 Plot E380 East end Transect B Close-up and overview photos of PEM1 vegetation community during June 2016 Plot D87 Plot A554 Plot C51 Plot H69 Close-up and overview photos of PEM2 Vegetation Community during June 2010 Plot A340 Plot D189 Plot E131 Plot E167 Close-up and overview photos of PEM2 vegetation community during June 2016 Plot H89 Plot H101 Plot G67 Plot Close-up and overview photos of PEM3 Vegetation Community during June 2010 Plot B43 Plot C63 Plot H216 Plot H136 Close-up and overview photos of PEM3 vegetation community during June 2016 Plot F138 Plot F118 Plot F138 Close-up and overview photos of PEM4 Vegetation Community During June 2010 Plot I226 Plot B154 Plot I255 Plot I255 Close-up and overview photos of PSS Vegetation Community during June 2010 West End Transect A Plot C568 Plot D364 Plot I150 Close-up and overview photos of PSS vegetation community during June 2016 Plot D625 Plot F64 Plot B644 Plot D567 Close-up and overview photos of Riparian 1 Vegetation Community during June 2010 Plot A640 East End Transect A East End Transect D East End Transect E Close-up and overview photos of Riparian 1 vegetation community during June 2016 Plot G102 Plot F246 Plot I410 Plot I410 Close-up and overview photos of Riparian 2 Vegetation Community during June 2010 East End Transect F Plot F281 Plot I378 Close-up and overview photos of Riparian 2 vegetation community during June 2016 ______________________________________________________________________________ -

Monografia-Rhamnus.Pdf
1 MINISTÉRIO DA SAÚDE MONOGRAFIA DA ESPÉCIE Rhamnus purshiana (CÁSCARA SAGRADA) Organização: Ministério da Saúde e Anvisa Fonte do Recurso: Ação 20K5 (DAF/SCTIE/MS)/2012 Brasília 2014 2 LISTA DE ILUSTRAÇÕES Figura 1 - Aspecto geral da espécie Rhamnus purshiana D.C. 1A: planta; 1B: casca do caule; 1C: folhas e flores; 1D: folhas e frutos 11 Figura 2 - Aspecto geral da droga vegetal (cascas) de Rhamnus purshiana D.C. 14 Figura 3 - Estruturas encontradas no pó da droga vegetal Rhamnus purshiana D.C. I e IA: fibras circundadas por prismas de oxalato de cálcio. 2: esclereides, mostrando um fragmento de camada de cristal (cr.) 3: fragmentos de cortiça e córtex em corte seccional, mostrando grupamento de cristais de oxalato de cálcio. 4: prismas e grupos de cristais de oxalato de cálcio. 5: vista superficial de fragmentos de células corticais. 6: fragmento de musgo. 7. Parte de um raio medular em corte tangencial longitudinal com parênquima contendo pontuações. 8. Floema em corte radial longitudinal mostrando um tubo crivado com placas crivadas (s.p.), parênquima contendo aglomerados de cristais de oxalato de cálcio e raio medular. 9: colênquima do córtex mostrando pontuações (pt.). 10: parênquima contendo grãos de amido. 11: células de floema parenquimatoso, mostrando excrescências na parede. 12: fragmentos de hepáticas. 16 Figura 4 - Principais metabólitos secundários encontrados em Rhamnus purshiana D.C. 28 Figura 5 - Esquema de biossíntese de derivados antracênicos 29 3 LISTA DE TABELAS Tabela 1 - Características da droga vegetal Rhamnus -

Investigation of Colorimetric Properties of Woolen Yarn Dyed with Rheum Ribes Plant Root Extract
Asian Journal of Chemistry Vol. 19, No. 5 (2007), 4043-4051 Investigation of Colorimetric Properties of Woolen Yarn Dyed with Rheum Ribes Plant Root Extract MENDERES KOYUNCU Department of Chemistry, Faculty of Arts and Sciences Yuzuncu Yil University, 65100 Van, Turkey Fax: (90)(432)2251415; Tel: (90)(432)2251136; E-mail: [email protected] The dyeing of wool yarn using Rheum ribes roots as natural dye has been studied in conventional method. The effects of dyeing show higher colour strength values obtained by the latter. Dyeing with Rheum ribes roots has been shown to give good dyeing results. The results of washing fastness properties of the dyed wool yarn were fair to good. CIELAB values have also been evaluated and discussed. Key Words: Natural dyes, Wool yarn, Rheum ribes, Dyeing, Fastness. INTRODUCTION Since prehistoric times, natural dyes have been used for many pur- poses such as the colouring of natural fibers wool, cotton and silk as well as fur and leather. The dyes were also used to colour cosmetic products and to produce inks, watercolours and artist's paints. The chemical structure of a dye molecule is divided in two parts i.e., the main chromophore and the auxochromes groups. The analysis of the natural dyes listed in Colour Index revealed that almost 50 % of all natural dyes used to colour textiles are flavonoid compounds. Most of the remain- ing natural dyes fall within 3 chemical classes viz., anthraquinones, naphtoquinones and indigoids1. The use of natural dyes to colour textiles declined rapidly after the discovery of synthetic dyes in 1856, until they were virtually unused by 1900. -

Tribe Species Secretory Structure Compounds Organ References Incerteae Sedis Alphitonia Sp. Epidermis, Idioblasts, Cavities
Table S1. List of secretory structures found in Rhamanaceae (excluding the nectaries), showing the compounds and organ of occurrence. Data extracted from the literature and from the present study (species in bold). * The mucilaginous ducts, when present in the leaves, always occur in the collenchyma of the veins, except in Maesopsis, where they also occur in the phloem. Tribe Species Secretory structure Compounds Organ References Epidermis, idioblasts, Alphitonia sp. Mucilage Leaf (blade, petiole) 12, 13 cavities, ducts Epidermis, ducts, Alphitonia excelsa Mucilage, terpenes Flower, leaf (blade) 10, 24 osmophores Glandular leaf-teeth, Flower, leaf (blade, Ceanothus sp. Epidermis, hypodermis, Mucilage, tannins 12, 13, 46, 73 petiole) idioblasts, colleters Ceanothus americanus Idioblasts Mucilage Leaf (blade, petiole), stem 74 Ceanothus buxifolius Epidermis, idioblasts Mucilage, tannins Leaf (blade) 10 Ceanothus caeruleus Idioblasts Tannins Leaf (blade) 10 Incerteae sedis Ceanothus cordulatus Epidermis, idioblasts Mucilage, tannins Leaf (blade) 10 Ceanothus crassifolius Epidermis; hypodermis Mucilage, tannins Leaf (blade) 10, 12 Ceanothus cuneatus Epidermis Mucilage Leaf (blade) 10 Glandular leaf-teeth Ceanothus dentatus Lipids, flavonoids Leaf (blade) (trichomes) 60 Glandular leaf-teeth Ceanothus foliosus Lipids, flavonoids Leaf (blade) (trichomes) 60 Glandular leaf-teeth Ceanothus hearstiorum Lipids, flavonoids Leaf (blade) (trichomes) 60 Ceanothus herbaceus Idioblasts Mucilage Leaf (blade, petiole), stem 74 Glandular leaf-teeth Ceanothus -

Lääkeluettelon Rohdokset , Liite 2. Luonnos Drogerna I
LÄÄKELUETTELON ROHDOKSET , LIITE 2. LUONNOS 1 DROGERNA I LÄKEMEDELSFÖRTECKNINGEN , BILAGA 2. UTKAST Latinankielinen nimi, Suomenkielinen nimi, Ruotsinkielinen nimi, Englanninkielinen nimi, Latinskt namn Finskt namn Svenskt namn Engelskt namn Abri semen (Abrus precatorius) Paternosterpapu Paternosterböna Abrus precatorius Paternosterpapu Paternosterböna Indian Liquorice Absinthii herba (Artemisia absinthium) Koiruoho, Mali Äkta malört Wormwood Acanthopanax senticosus (=Eleutherococcus s.) Venäjänjuuri Ryskrot Acanthopanaxsessilifloru s (=Eleutherococcus s.) Kiinanaralehti Kinesisk stickaralia Aconiti tuber (Aconitum napellus) Ukonhatunmukula Stormhatts knöl Common Monkshood / Mouse Aconitum napellus Aitoukonhattu Stormhatt bane / Frair's Cap Aconitum sp. Ukonhatut Stormhatt Monkshood Calamus / Cinnamon Sedge / Acorus calamus Rohtokalmojuuri Kalmus Sweet Flag Adonidis herba (Adonis vernalis) Adonisyrtti Adonisört Pheasant´s eye Adonis vernalis Kevätruusuleinikki Våradonis Yellow Pheasant's Eye Aegle marmelos Belahedelmä Belafrukt Indian bael Common Horse Chestnut / Aesculus hippocastanum Hevoskastanja Hästkastanj Horse Chestnut / Buckeye Chaste berry / Chaste Tree / Agni casti fructus Hemp Tree / Chastelamb / (Vitex agnus castus) Siveydenpuun hedelmä Kyskhetsträd, Munkpeppar Monk´s pepper Aloe barbadensis Barbados aloes / Mediterranean (=Aloe vera) Lääkeaaloe Såraloe aloes Aloe capensis Kapin aaloe Kap-Aloe Cape aloes Aloe sp. Aaloet Aloe Aloes Aloe vera Barbados aloes / Mediterranean (=Aloe barbadensis) Lääkeaaloe Såraloe aloes Amanita