Effects of Acclimation Temperature on Critical Thermal Limits and Swimming Performance of the State-Endangered Bigeye Chub Hybopsis Amblops
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Indiana Species April 2007
Fishes of Indiana April 2007 The Wildlife Diversity Section (WDS) is responsible for the conservation and management of over 750 species of nongame and endangered wildlife. The list of Indiana's species was compiled by WDS biologists based on accepted taxonomic standards. The list will be periodically reviewed and updated. References used for scientific names are included at the bottom of this list. ORDER FAMILY GENUS SPECIES COMMON NAME STATUS* CLASS CEPHALASPIDOMORPHI Petromyzontiformes Petromyzontidae Ichthyomyzon bdellium Ohio lamprey lampreys Ichthyomyzon castaneus chestnut lamprey Ichthyomyzon fossor northern brook lamprey SE Ichthyomyzon unicuspis silver lamprey Lampetra aepyptera least brook lamprey Lampetra appendix American brook lamprey Petromyzon marinus sea lamprey X CLASS ACTINOPTERYGII Acipenseriformes Acipenseridae Acipenser fulvescens lake sturgeon SE sturgeons Scaphirhynchus platorynchus shovelnose sturgeon Polyodontidae Polyodon spathula paddlefish paddlefishes Lepisosteiformes Lepisosteidae Lepisosteus oculatus spotted gar gars Lepisosteus osseus longnose gar Lepisosteus platostomus shortnose gar Amiiformes Amiidae Amia calva bowfin bowfins Hiodonotiformes Hiodontidae Hiodon alosoides goldeye mooneyes Hiodon tergisus mooneye Anguilliformes Anguillidae Anguilla rostrata American eel freshwater eels Clupeiformes Clupeidae Alosa chrysochloris skipjack herring herrings Alosa pseudoharengus alewife X Dorosoma cepedianum gizzard shad Dorosoma petenense threadfin shad Cypriniformes Cyprinidae Campostoma anomalum central stoneroller -

Species Status Assessment (SSA) Report for the Ozark Chub (Erimystax Harryi) Version 1.2
Species Status Assessment (SSA) Report for the Ozark Chub (Erimystax harryi) Version 1.2 Ozark chub (Photo credit: Dustin Lynch, Arkansas Natural Heritage Commission) August 2019 U.S. Fish and Wildlife Service - Arkansas Ecological Services Field Office This document was prepared by Alyssa Bangs (U. S. Fish and Wildlife Service (USFWS) – Arkansas Ecological Services Field Office), Bryan Simmons (USFWS—Missouri Ecological Services Field Office), and Brian Evans (USFWS –Southeast Regional Office). We greatly appreciate the assistance of Jeff Quinn (Arkansas Game and Fish Commission), Brian Wagner (Arkansas Game and Fish Commission), and Jacob Westhoff (Missouri Department of Conservation) who provided helpful information and review of the draft document. We also thank the peer reviewers, who provided helpful comments. Suggested reference: U.S. Fish and Wildlife Service. 2019. Species status assessment report for the Ozark chub (Erimystax harryi). Version 1.2. August 2019. Atlanta, GA. CONTENTS Chapter 1: Executive Summary 1 1.1 Background 1 1.2 Analytical Framework 1 CHAPTER 2 – Species Information 4 2.1 Taxonomy and Genetics 4 2.2 Species Description 5 2.3 Range 6 Historical Range and Distribution 6 Current Range and Distribution 8 2.4 Life History Habitat 9 Growth and Longevity 9 Reproduction 9 Feeding 10 CHAPTER 3 –Factors Influencing Viability and Current Condition Analysis 12 3.1 Factors Influencing Viability 12 Sedimentation 12 Water Temperature and Flow 14 Impoundments 15 Water Chemistry 16 Habitat Fragmentation 17 3.2 Model 17 Analytical -

Reproductive Timing of the Largescale Stoneroller, Campostoma Oligolepis, in the Flint River, Alabama
REPRODUCTIVE TIMING OF THE LARGESCALE STONEROLLER, CAMPOSTOMA OLIGOLEPIS, IN THE FLINT RIVER, ALABAMA by DANA M. TIMMS A THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in The Department of Biological Sciences to The School of Graduate Studies of The University of Alabama in Huntsville HUNTSVILLE, ALABAMA 2017 ACKNOWLEDGEMENTS I would like to thank my advisor Dr. Bruce Stallsmith for all his guidance on this project and greater dedication to raising awareness to Alabama’s river ecosystems. I am grateful to my other committee members, Dr. Gordon MacGregor and Dr. Debra Moriarity also from UAH. Thanks to everyone who braved the weather and elements on collecting trips: Tiffany Bell, Austin Riley, Chelsie Smith, and Joshua Mann. I would like to thank Megan McEown, Corinne Peacher, and Bonnie Ferguson for dedicating long hours in the lab. Special thanks to Matthew Moore who assisted in collections, lab work, and data processing. Most of all, I would like to thank my husband, Patrick, for his love and encouragement in all my endeavors. v TABLE OF CONTENTS Page • List of Figures viii • List of Tables x • CHAPTER ONE: Introduction 1 o Context 1 o Campostoma oligolepis Taxonomy 3 o History of Campostoma oligolepis 4 ▪ Campostoma oligolepis in the South 6 ▪ Campostoma Hybridization 8 ▪ Campostoma Ranges and Species Differentiation 9 ▪ Life History 12 ▪ Reproduction 12 o Purpose and Hypothesis 15 • CHAPTER TWO: Methodology 17 o Laboratory Analysis 19 o Reproductive Data 21 o Ovary and Oocyte Staging 22 o Statistical Analysis 22 • CHAPTER THREE: Results 27 o Reproductive Data 29 ▪ Ovary and Oocyte Development 32 ▪ Testicular Development 39 vi • CHAPTER FOUR: Discussion 40 o Study Limitations 40 o Lateral Line Scale Count 41 o Reproductive Cues and Environmental Influences 42 o Multiple-spawners 42 o Asymmetry of Ovaries 42 o Bourgeois Males 43 o Campostoma variability 44 o Conclusion 45 • WORKS CITED 46 vii LIST OF FIGURES Page • 1.1 Campostoma oligolepis, Largescale Stoneroller, specimens from the Flint River, Alabama. -

Distribution Changes of Small Fishes in Streams of Missouri from The
Distribution Changes of Small Fishes in Streams of Missouri from the 1940s to the 1990s by MATTHEW R. WINSTON Missouri Department of Conservation, Columbia, MO 65201 February 2003 CONTENTS Page Abstract……………………………………………………………………………….. 8 Introduction…………………………………………………………………………… 10 Methods……………………………………………………………………………….. 17 The Data Used………………………………………………………………… 17 General Patterns in Species Change…………………………………………... 23 Conservation Status of Species……………………………………………….. 26 Results………………………………………………………………………………… 34 General Patterns in Species Change………………………………………….. 30 Conservation Status of Species……………………………………………….. 46 Discussion…………………………………………………………………………….. 63 General Patterns in Species Change………………………………………….. 53 Conservation Status of Species………………………………………………. 63 Acknowledgments……………………………………………………………………. 66 Literature Cited……………………………………………………………………….. 66 Appendix……………………………………………………………………………… 72 FIGURES 1. Distribution of samples by principal investigator…………………………. 20 2. Areas of greatest average decline…………………………………………. 33 3. Areas of greatest average expansion………………………………………. 34 4. The relationship between number of basins and ……………………….. 39 5. The distribution of for each reproductive group………………………... 40 2 6. The distribution of for each family……………………………………… 41 7. The distribution of for each trophic group……………...………………. 42 8. The distribution of for each faunal region………………………………. 43 9. The distribution of for each stream type………………………………… 44 10. The distribution of for each range edge…………………………………. 45 11. Modified -

Pennsylvania Fishes IDENTIFICATION GUIDE
Pennsylvania Fishes IDENTIFICATION GUIDE WATERSHEDS SPECIES STATUS E O G P S D Editor’s Note: During 2018, Carps and Minnows (Family Cyprinidae) Pennsylvania Angler & Boater Central Stoneroller (Campostoma anomalum) N N N N N N magazine will feature select Goldfish (Carassius auratus) I I I I I common fishes of Pennsylvania Northern Redbelly Dace (Chrosomus eos) EN N N in each issue, providing scientific Southern Redbelly Dace (Chrosomus erythrogaster) TH N N names and the status of fishes in Mountain Redbelly Dace (Chrosomus oreas) I Redside Dace (Clinostomus elongatus) N N N X or introduced into Pennsylvania’s Rosyside Dace (Clinostomus funduloides) N N N major watersheds. Grass Carp (Ctenopharyngodon idella) I I I I I I The table to the left denotes any Satinfin Shiner (Cyprinella analostana) N N N known occurrence. Spotfin Shiner (Cyprinella spiloptera) N N N N N Steelcolor Shiner (Cyprinella whipplei) N Common Carp (Cyprinus carpio) I I I I I Streamline Chub (Erimystax dissimilis) N Gravel Chub (Erimystax x-punctatus) EN N Species Status Tonguetied Minnow (Exoglossum laurae) N N Cutlip Minnow (Exoglossum maxillingua) N N N EN = Endangered Brassy Minnow (Hybognathus hankinsoni) X TH = Threatened Eastern Silvery Minnow (Hybognathus regius) N N N Bigeye Chub (Hybopsis amblops) N N C = Candidate Bigmouth Shiner (Hybopsis dorsalis) TH N EX = Believed extirpated Ide (Leuciscus idus) I I Striped Shiner (Luxilus chrysocephalus) N N DL = Delisted (removed from the Common Shiner (Luxilus cornutus) N N N N N N endangered, threatened or candidate -
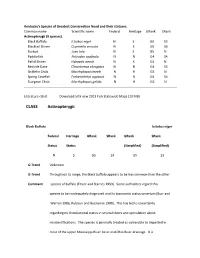
CLASS Actinopterygii
Kentucky's Species of Greatest Conservation Need and their statuses. Common name Scientific name Federal Heritage GRank SRank Actinopterygii (9 species). Black Buffalo Ictiobus niger N S G5 S3 Blacktail Shiner Cyprinella venusta N S G5 S3 Burbot Lota lota N S G5 N Paddlefish Polyodon spathula N N G4 S4 Pallid Shiner Hybopsis amnis N X G4 N Redside Dace Clinostomus elongatus N N G4 S3 Sicklefin Chub Macrhybopsis meeki N H G3 N Spring Cavefish Forbesichthys agassizii N N G4 S4 Sturgeon Chub Macrhybopsis gelida N H G3 N Literature cited Download all 9 new 2013 Fish Statewide Maps (10 MB) CLASS Actinopterygii Black Buffalo Ictiobus niger Federal Heritage GRank SRank GRank SRank Status Status (Simplified) (Simplified) N S G5 S3 G5 S3 G-Trend Unknown G-Trend Throughout its range, the black buffalo appears to be less common than the other Comment species of buffalo (Etnier and Starnes 1993). Some authorities regard this species to be inadequately diagnosed and its taxonomic status uncertain (Burr and Warren 1986, Robison and Buchanan 1988). This has led to uncertainty regarding its distributional status in several states and speculation about misidentifications. The species is generally treated as vulnerable to imperiled in most of the upper Mississippi River basin and Ohio River drainage. It is considered secure in only a few states in the middle and lower Mississippi River basin, although records in the Gulf Slope drainages in Texas and New Mexico are thought to potentially be based on misidentifications or introductions (Etnier and Starnes 1993, Natureserve 2008, Shute 1980). S-Trend Unknown S-Trend Burr and Warren (1986) regarded this species as sporadic and rare in rivers and Comment reservoirs in western Kentucky, and sporadic in the main channels of the Mississippi and Ohio rivers. -

Current Status of Native Fish Species in Kansas
TRANSACTIONS OF THE KANSAS Vol. 108, no. 1/2 ACADEMY OF SCIENCE p. 32-46 (2005) Current status of native fish species in Kansas STEPHEN G. H ASLOUER,1 MARK E. EBERLE,2 DAVID R. EDDS,3 KEITH B. GIDO,4 CHRIS S. MAMMOLITI,5 JAMES R. TRIPLETT,6 JOSEPH T. COLLINS,7 DONALD A. DISTLER,8 DONALD G. H UGGINS7 AND WILLIAM J. STARK2 1. Division of Environment, Kansas Department of Health and Environment, 1000 SW Jackson St., Topeka, Kansas 66612 ([email protected]) 2. Department of Biological Sciences, Fort Hays State University, Hays, Kansas 67601 3. Department of Biological Sciences, Emporia State University, Emporia, Kansas 66801 4. Division of Biology, Kansas State University, Manhattan, Kansas 66506 5. Tetra Tech EM Inc., 1200 SW Executive Drive, Topeka, Kansas 66617 6. Department of Biology, Pittsburg State University, Pittsburg, Kansas 66762 7. Kansas Biological Survey, University of Kansas, Lawrence, Kansas 66047 8. Department of Biological Sciences, Wichita State University, Wichita, Kansas 67260 A re-evaluation of the status of fishes in Kansas suggests that 54 of the 116 native species should be assigned special conservation status due to substantial declines in distribution or abundance and/or their rarity in the state. Nine species are recommended for retention in their existing status of endangered, threatened, or species in need of conservation. We recommend elevated conservation status for 44 additional species, and provide information on trends in distribution and abundance for these taxa. A single species, the Arkansas River Shiner, Notropis girardi, is considered to be extirpated recently from Kansas. Keywords: fishes, endangered, threatened, conservation, Kansas. -

Bear Creek System for Fish Species of Moderate to Highest Conservation Concern: Report of Results for 2007-08
GEOLOGICAL SURVEY OF ALABAMA Berry H. (Nick) Tew, Jr. State Geologist Water Investigations Program Patrick E. O’Neil Director SURVEY OF THE BEAR CREEK SYSTEM FOR FISH SPECIES OF MODERATE TO HIGHEST CONSERVATION CONCERN: REPORT OF RESULTS FOR 2007-08 OPEN-FILE REPORT 0901 by Thomas E. Shepard, Stuart W. McGregor, Patrick E. O'Neil, Maurice F. Mettee, J. Brett Smith, Cal C. Johnson, and Josh H. Hunter Prepared in cooperation with the Alabama Department of Conservation and Natural Resources Tuscaloosa, Alabama 2009 CONTENTS Abstract ............................................................ 1 Acknowledgments .................................................... 1 Introduction.......................................................... 1 Study area .......................................................... 4 Methods ............................................................ 6 Results and discussion................................................ 11 Study plan for 2009 .................................................. 26 References cited..................................................... 29 Appendix: Collection results for fish samples in the Bear Creek system, 2007-08 . 32 ILLUSTRATIONS 1. Sampling stations in the Bear Creek system, 2007-08 . 5 2. Sampling stations where the brindled madtom, Noturus miurus, was collected in the Bear Creek system, 2007-08 ...................................... 16 3. Sampling stations where the bandfin darter, Etheostoma zonisteum, was collected in the Bear Creek system, 2007-08 .................................. -

Freshwater Fish
Common Name: American eel SGCN – High Priority Scientific Name: Anguilla rostrata Taxon: Freshwater Fish Federal Status: Candidate Natural Heritage Program Rank: New York Status: Not Listed Global: G4 New York: S3 Tracked: No Synopsis: The American eel, Anguilla rostrata lives in nearshore areas of lakes and streams with various bottom types, including rocks. The American eel has a very large range in the Atlantic Ocean and estuaries and rivers of the Atlantic and Gulf coasts of the United States and southeastern Canada, as well as much of the Mississippi River basin and the West Indies and Caribbean regions. Individuals travel to ocean spawning areas near the end of its life. The American eel is considered a single stock since all mature eels from the entire range migrate the Sargasso Sea to spawn. They only spawn once during their lifetime, making it especially difficult to protect this species. The American eel is native to 17 of 18 watersheds in New York and is still found in 15. Its New York range has been extended into the Erie and upper Genesee watersheds, while the Erie is the only one where it is entirely non-native. It continues to be found in many of the areas previously known in the Long Island, Delaware, and Lower Hudson watersheds but has declined to near absence in all the others. Extensive information on New York’s inland population is reported by Dittman et al. (2010a). A 2010 petition seeking protection of the American eel under the Endangered Species Act resulted in a 90-day finding in 2011, and an extensive status review is now being conducted to determine whether federal protection is warranted (USFWS 2011). -

Spring Creek Fishes
Spring Creek Fishes David L. McNeely, Ph.D. This is a list of fish species known by me to occur in Spring Creek and its tributaries as of August 2011 or before. Forty-one species include specimens listed in museum records (Sam Noble Oklahoma Museum of Natural History, Oklahoma State University, University of Kansas, University of Michigan) or reported in publications. Six additional species as noted in the list have been observed in the field by competent authorities but no published records or catalogued museum specimens are known to me. Besides myself, Robert Heth (Associate Professor of Biology, Missouri Southern State University), David Martinez (U.S. Fish and Wildlife Service), and W.J. Matthews (Professor of Zoology, University of Oklahoma) have extensive knowledge of the Spring Creek fish fauna. Abundance values: a = abundant, many individuals at a majority of locations in all sections of the stream. Most visitors should be able to observe these species. c = common, many individuals at some locations, though not in all sections of the stream. Attentive visitors should be able to observe these species. p = present, may be common at a few locations, or may occur at many locations but not in substantial numbers. Very attentive visitors who work to do so may be able to observe these species. u = uncommon, present at few locations in small numbers. Most visitors will not observe these species. Even attentive individuals may fail to find them. r = rare, only known from a very small number of locations, and only found during intensive ichthylogical work by dedicated workers. May be extirpated from some reported localities. -
![Reproductive Biology of [I]Hybopsis Amblops[I], the Bigeye Chub, in The](https://docslib.b-cdn.net/cover/7182/reproductive-biology-of-i-hybopsis-amblops-i-the-bigeye-chub-in-the-4787182.webp)
Reproductive Biology of [I]Hybopsis Amblops[I], the Bigeye Chub, in The
Reproductive biology of Hybopsis amblops, the Bigeye Chub, in the Flint River of Alabama Background. The purpose of this study was to establish a reproductive schedule and examine reproductive traits that shape fecundity of the Bigeye Chub, Hybopsis amblops Cyprinidae, in the Flint River system of north Alabama. Methods. Life history traits associated with reproduction, growth, and maturation were assessed. Fish collections were made monthly from August, 2013, through July, 2014. Results. The Bigeye Chub in Alabama primarily spawns in April and May as indicated by gonadosomatic index (GSI), ovarian condition and clutch size. Average GSI values began to rise in February, peaked in April and May at over 13% for females and 1.6% for males, and showed a steep decline from May to June for both sexes. Average clutch size was highest in April at 812. Diameter of the most mature oocyte stage averaged 0.74 mm, relatively small compared to other cyprinids found in the Flint River. Discussion. The Bigeye Chub’s relatively large clutch size as a measure of fecundity places the species intermediate between opportunistic and periodic in the trilateral life history scheme of Winemiller and Rose. The species is apparently responding to a flow regime with a defined seasonality as well as predictability of flow and resources. PeerJ Preprints | https://doi.org/10.7287/peerj.preprints.26719v3 | CC BY 4.0 Open Access | rec: 3 Jul 2019, publ: 4 Jul 2019 Reproductive biology of Hybopsis amblops, the Bigeye Chub, in the Flint River of Alabama Crissy L Tarver 1 , Bruce Stallsmith Corresp. 1 1 Biological Sciences, University of Alabama - Huntsville, Huntsville, Alabama, United States Corresponding Author: Bruce Stallsmith Email address: [email protected] Background. -

097-27MS 2408-Print
Transactions of the Illinois State Academy of Science received 5/24/04 (2004), Volume 97, #3&4, pp. 255-257 accepted 1/16/05 Range Expansion of the State-Endangered Bigeye Chub Hybopsis amblops (Rafinesque) in Illinois Jeremy S. Tiemann1*, Mike E. Retzer1, and Bernadette L. Tiemann2 1Illinois Natural History Survey, Center for Biodiversity 607 E. Peabody Drive, Champaign, IL 61820 2First School, 1403 E. Oak Street, Mahomet, IL 61853 *Correspondence: [email protected] ABSTRACT One specimen of the state-endangered bigeye chub Hybopsis amblops (Rafinesque) was collected in Little Beaver Creek, Kankakee County, Illinois, on 2 May 2004, and depos- ited at the Illinois Natural History Survey Fish Collection, Champaign (INHS 98619). This specimen represents a range expansion into a new river drainage. Key Words: Bigeye chub, Hybopsis amblops, Little Beaver Creek, endangered species The bigeye chub Hybopsis amblops (Rafinesque) is state-listed as endangered in Illinois (IESPB, 1999). On 2 May 2004, two of us (JST and BLT) collected a 40.2 mm SL (50.7 mm TL) female H. amblops with latent ovaries by common-sense seining in Little Beaver Creek (Iroquois River - Kankakee River drainage), 2.0 km W Leesville, Kankakee County, Illinois. This specimen possessed maxillary barbels, an eye diameter slightly greater than length of snout, and a pronounced black lateral stripe extending from tip of snout to caudal peduncle, fitting Smith’s (1979) description of H. amblops. This speci- men was collected in a small school (five individuals) of ironcolor shiners Notropis cha- lybaeus (Cope), another state-listed species (IESPB, 1999). The H.