Specific Heat of Solids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lab: Specific Heat
Lab: Specific Heat Summary Students relate thermal energy to heat capacity by comparing the heat capacities of different materials and graphing the change in temperature over time for a specific material. Students explore the idea of insulators and conductors in an attempt to apply their knowledge in real world applications, such as those that engineers would. Engineering Connection Engineers must understand the heat capacity of insulators and conductors if they wish to stay competitive in “green” enterprises. Engineers who design passive solar heating systems take advantage of the natural heat capacity characteristics of materials. In areas of cooler climates engineers chose building materials with a low heat capacity such as slate roofs in an attempt to heat the home during the day. In areas of warmer climates tile roofs are popular for their ability to store the sun's heat energy for an extended time and release it slowly after the sun sets, to prevent rapid temperature fluctuations. Engineers also consider heat capacity and thermal energy when they design food containers and household appliances. Just think about your aluminum soda can versus glass bottles! Grade Level: 9-12 Group Size: 4 Time Required: 90 minutes Activity Dependency :None Expendable Cost Per Group : US$ 5 The cost is less if thermometers are available for each group. NYS Science Standards: STANDARD 1 Analysis, Inquiry, and Design STANDARD 4 Performance indicator 2.2 Keywords: conductor, energy, energy storage, heat, specific heat capacity, insulator, thermal energy, thermometer, thermocouple Learning Objectives After this activity, students should be able to: Define heat capacity. Explain how heat capacity determines a material's ability to store thermal energy. -

Density of States Information from Low Temperature Specific Heat
JOURNAL OF RESE ARC H of th e National Bureau of Standards - A. Physics and Chemistry Val. 74A, No.3, May-June 1970 Density of States I nformation from Low Temperature Specific Heat Measurements* Paul A. Beck and Helmut Claus University of Illinois, Urbana (October 10, 1969) The c a lcul ati on of one -electron d ensit y of s tate va lues from the coeffi cient y of the te rm of the low te mperature specifi c heat lin ear in te mperature is compli cated by many- body effects. In parti c ul ar, the electron-p honon inte raction may enhance the measured y as muc h as tw ofo ld. The e nha nce me nt fa ctor can be eva luat ed in the case of supe rconducting metals and a ll oys. In the presence of magneti c mo ments, add it ional complicati ons arise. A magneti c contribution to the measured y was ide ntifi e d in the case of dilute all oys and a lso of concentrated a lJ oys wh e re parasiti c antife rromagnetis m is s upe rim posed on a n over-a ll fe rromagneti c orde r. No me thod has as ye t bee n de vised to e valu ate this magne ti c part of y. T he separati on of the te mpera ture- li near term of the s pec ifi c heat may itself be co mpli cated by the a ppearance of a s pecific heat a no ma ly due to magneti c cluste rs in s upe rpa ramagneti c or we ak ly ferromagneti c a ll oys. -

Compression of an Ideal Gas with Temperature Dependent Specific
Session 3666 Compression of an Ideal Gas with Temperature-Dependent Specific Heat Capacities Donald W. Mueller, Jr., Hosni I. Abu-Mulaweh Engineering Department Indiana University–Purdue University Fort Wayne Fort Wayne, IN 46805-1499, USA Overview The compression of gas in a steady-state, steady-flow (SSSF) compressor is an important topic that is addressed in virtually all engineering thermodynamics courses. A typical situation encountered is when the gas inlet conditions, temperature and pressure, are known and the compressor dis- charge pressure is specified. The traditional instructional approach is to make certain assumptions about the compression process and about the gas itself. For example, a special case often consid- ered is that the compression process is reversible and adiabatic, i.e. isentropic. The gas is usually ÊÌ considered to be ideal, i.e. ÈÚ applies, with either constant or temperature-dependent spe- cific heat capacities. The constant specific heat capacity assumption allows for direct computation of the discharge temperature, while the temperature-dependent specific heat assumption does not. In this paper, a MATLAB computer model of the compression process is presented. The substance being compressed is air which is considered to be an ideal gas with temperature-dependent specific heat capacities. Two approaches are presented to describe the temperature-dependent properties of air. The first approach is to use the ideal gas table data from Ref. [1] with a look-up interpolation scheme. The second approach is to use a curve fit with the NASA Lewis coefficients [2,3]. The computer model developed makes use of a bracketing–bisection algorithm [4] to determine the temperature of the air after compression. -
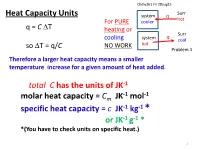
Total C Has the Units of JK-1 Molar Heat Capacity = C Specific Heat Capacity
Chmy361 Fri 28aug15 Surr Heat Capacity Units system q hot For PURE cooler q = C ∆T heating or q Surr cooling system cool ∆ C NO WORK hot so T = q/ Problem 1 Therefore a larger heat capacity means a smaller temperature increase for a given amount of heat added. total C has the units of JK-1 -1 -1 molar heat capacity = Cm JK mol specific heat capacity = c JK-1 kg-1 * or JK-1 g-1 * *(You have to check units on specific heat.) 1 Which has the higher heat capacity, water or gold? Molar Heat Cap. Specific Heat Cap. -1 -1 -1 -1 Cm Jmol K c J kg K _______________ _______________ Gold 25 129 Water 75 4184 2 From Table 2.2: 100 kg of water has a specific heat capacity of c = 4.18 kJ K-1kg-1 What will be its temperature change if 418 kJ of heat is removed? ( Relates to problem 1: hiker with wet clothes in wind loses body heat) q = C ∆T = c x mass x ∆T (assuming C is independent of temperature) ∆T = q/ (c x mass) = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = -418/418 = -1.0 K Note: Units are very important! Always attach units to each number and make sure they cancel to desired unit Problems you can do now (partially): 1b, 15, 16a,f, 19 3 A Detour into the Microscopic Realm pext Pressure of gas, p, is caused by enormous numbers of collisions on the walls of the container. -

Entropy Determination of Single-Phase High Entropy Alloys with Different Crystal Structures Over a Wide Temperature Range
entropy Article Entropy Determination of Single-Phase High Entropy Alloys with Different Crystal Structures over a Wide Temperature Range Sebastian Haas 1, Mike Mosbacher 1, Oleg N. Senkov 2 ID , Michael Feuerbacher 3, Jens Freudenberger 4,5 ID , Senol Gezgin 6, Rainer Völkl 1 and Uwe Glatzel 1,* ID 1 Metals and Alloys, University Bayreuth, 95447 Bayreuth, Germany; [email protected] (S.H.); [email protected] (M.M.); [email protected] (R.V.) 2 UES, Inc., 4401 Dayton-Xenia Rd., Dayton, OH 45432, USA; [email protected] 3 Institut für Mikrostrukturforschung, Forschungszentrum Jülich, 52425 Jülich, Germany; [email protected] 4 Leibniz Institute for Solid State and Materials Research Dresden (IFW Dresden), 01069 Dresden, Germany; [email protected] 5 TU Bergakademie Freiberg, Institut für Werkstoffwissenschaft, 09599 Freiberg, Germany 6 NETZSCH Group, Analyzing & Testing, 95100 Selb, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-921-55-5555 Received: 31 July 2018; Accepted: 20 August 2018; Published: 30 August 2018 Abstract: We determined the entropy of high entropy alloys by investigating single-crystalline nickel and five high entropy alloys: two fcc-alloys, two bcc-alloys and one hcp-alloy. Since the configurational entropy of these single-phase alloys differs from alloys using a base element, it is important to quantify ◦ the entropy. Using differential scanning calorimetry, cp-measurements are carried out from −170 C to the materials’ solidus temperatures TS. From these experiments, we determined the thermal entropy and compared it to the configurational entropy for each of the studied alloys. -

THERMOCHEMISTRY – 2 CALORIMETRY and HEATS of REACTION Dr
THERMOCHEMISTRY – 2 CALORIMETRY AND HEATS OF REACTION Dr. Sapna Gupta HEAT CAPACITY • Heat capacity is the amount of heat needed to raise the temperature of the sample of substance by one degree Celsius or Kelvin. q = CDt • Molar heat capacity: heat capacity of one mole of substance. • Specific Heat Capacity: Quantity of heat needed to raise the temperature of one gram of substance by one degree Celsius (or one Kelvin) at constant pressure. q = m s Dt (final-initial) • Measured using a calorimeter – it absorbed heat evolved or absorbed. Dr. Sapna Gupta/Thermochemistry-2-Calorimetry 2 EXAMPLES OF SP. HEAT CAPACITY The higher the number the higher the energy required to raise the temp. Dr. Sapna Gupta/Thermochemistry-2-Calorimetry 3 CALORIMETRY: EXAMPLE - 1 Example: A piece of zinc weighing 35.8 g was heated from 20.00°C to 28.00°C. How much heat was required? The specific heat of zinc is 0.388 J/(g°C). Solution m = 35.8 g s = 0.388 J/(g°C) Dt = 28.00°C – 20.00°C = 8.00°C q = m s Dt 0.388 J q 35.8 g 8.00C = 111J gC Dr. Sapna Gupta/Thermochemistry-2-Calorimetry 4 CALORIMETRY: EXAMPLE - 2 Example: Nitromethane, CH3NO2, an organic solvent burns in oxygen according to the following reaction: 3 3 1 CH3NO2(g) + /4O2(g) CO2(g) + /2H2O(l) + /2N2(g) You place 1.724 g of nitromethane in a calorimeter with oxygen and ignite it. The temperature of the calorimeter increases from 22.23°C to 28.81°C. -

Chapter 5. Calorimetiric Properties
Chapter 5. Calorimetiric Properties (1) Specific and molar heat capacities (2) Latent heats of crystallization or fusion A. Heat Capacity - The specific heat capacity is the heat which must be added per kg of a substance to raise the temperature by one degree 1. Specific heat capacity at const. Volume ∂U c = (J/kg· K) v ∂ T v 2. Specific heat capacity at cont. pressure ∂H c = (J/kg· K) p ∂ T p 3. molar heat capacity at constant volume Cv = Mcv (J/mol· K) 4. molar heat capacity at constat pressure ∂H C = M = (J/mol· K) p cp ∂ T p Where H is the enthalpy per mol ◦ Molar heat capacity of solid and liquid at 25℃ s l - Table 5.1 (p.111)에 각각 group 의 Cp (Satoh)와 Cp (Show)에 대한 data 를 나타내었다. l s - Table 5.2 (p.112,113)에 각 polymer 에 대한 Cp , Cp 값들을 나타내었다. - (Ex 5.1)에 polypropylene 의 Cp 를 구하는 것을 나타내었다. - ◦ Specific heat as a function of temperature - 그림 5.1 에 polypropylene 의 molar heat capacity 에 대한 그림을 나타 내었다. ◦ The slopes of the heat capacity for solid polymer dC s 1 p = × −3 s 3 10 C p (298) dt dC l 1 p = × −3 l 1.2 10 C p (298) dt l s (see Table 5.3) for slope of the Cp , Cp ◦ 식 (5.1)과 (5.2)에 온도에 따른 Cp 값 계산 (Ex) calculate the heat capacity of polypropylene with a degree of crystallinity of 30% at 25℃ (p.110) (sol) by the addition of group contributions (see table 5.1) s l Cp (298K) Cp (298K) ( -CH2- ) 25.35 30.4(from p.111) ( -CH- ) 15.6 20.95 ( -CH3 ) 30.9 36.9 71.9 88.3 ∴ Cp(298K) = 0.3 (71.9J/mol∙ K) + 0.7(88.3J/mol∙ K) = 83.3 (J/mol∙ K) - It is assumed that the semi crystalline polymer consists of an amorphous fraction l s with heat capacity Cp and a crystalline Cp P 116 Theoretical Background P 117 The increase of the specific heat capacity with temperature depends on an increase of the vibrational degrees of freedom. -

Module P7.4 Specific Heat, Latent Heat and Entropy
FLEXIBLE LEARNING APPROACH TO PHYSICS Module P7.4 Specific heat, latent heat and entropy 1 Opening items 4 PVT-surfaces and changes of phase 1.1 Module introduction 4.1 The critical point 1.2 Fast track questions 4.2 The triple point 1.3 Ready to study? 4.3 The Clausius–Clapeyron equation 2 Heating solids and liquids 5 Entropy and the second law of thermodynamics 2.1 Heat, work and internal energy 5.1 The second law of thermodynamics 2.2 Changes of temperature: specific heat 5.2 Entropy: a function of state 2.3 Changes of phase: latent heat 5.3 The principle of entropy increase 2.4 Measuring specific heats and latent heats 5.4 The irreversibility of nature 3 Heating gases 6 Closing items 3.1 Ideal gases 6.1 Module summary 3.2 Principal specific heats: monatomic ideal gases 6.2 Achievements 3.3 Principal specific heats: other gases 6.3 Exit test 3.4 Isothermal and adiabatic processes Exit module FLAP P7.4 Specific heat, latent heat and entropy COPYRIGHT © 1998 THE OPEN UNIVERSITY S570 V1.1 1 Opening items 1.1 Module introduction What happens when a substance is heated? Its temperature may rise; it may melt or evaporate; it may expand and do work1—1the net effect of the heating depends on the conditions under which the heating takes place. In this module we discuss the heating of solids, liquids and gases under a variety of conditions. We also look more generally at the problem of converting heat into useful work, and the related issue of the irreversibility of many natural processes. -

Atkins' Physical Chemistry
Statistical thermodynamics 2: 17 applications In this chapter we apply the concepts of statistical thermodynamics to the calculation of Fundamental relations chemically significant quantities. First, we establish the relations between thermodynamic 17.1 functions and partition functions. Next, we show that the molecular partition function can be The thermodynamic functions factorized into contributions from each mode of motion and establish the formulas for the 17.2 The molecular partition partition functions for translational, rotational, and vibrational modes of motion and the con- function tribution of electronic excitation. These contributions can be calculated from spectroscopic data. Finally, we turn to specific applications, which include the mean energies of modes of Using statistical motion, the heat capacities of substances, and residual entropies. In the final section, we thermodynamics see how to calculate the equilibrium constant of a reaction and through that calculation 17.3 Mean energies understand some of the molecular features that determine the magnitudes of equilibrium constants and their variation with temperature. 17.4 Heat capacities 17.5 Equations of state 17.6 Molecular interactions in A partition function is the bridge between thermodynamics, spectroscopy, and liquids quantum mechanics. Once it is known, a partition function can be used to calculate thermodynamic functions, heat capacities, entropies, and equilibrium constants. It 17.7 Residual entropies also sheds light on the significance of these properties. 17.8 Equilibrium constants Checklist of key ideas Fundamental relations Further reading Discussion questions In this section we see how to obtain any thermodynamic function once we know the Exercises partition function. Then we see how to calculate the molecular partition function, and Problems through that the thermodynamic functions, from spectroscopic data. -

Mixed Valence Driven Heavy-Fermion Behavior and Superconductivity in Kni2se2
Mixed Valence Driven Heavy-Fermion Behavior and Superconductivity in KNi2Se2 James R. Neilson,1,2† Anna Llobet,3 Andreas V. Stier,2 Liang Wu,2 Jiajia Wen,2 Jing Tao,4 Yimei Zhu,4 Zlatko B. Tesanovic,2 N. P. Armitage,2 and Tyrel M. McQueen1,2* 1 Department of Chemistry, Johns Hopkins University, Baltimore MD. 2 Institute for Quantum Matter, and Department of Physics and Astronomy, Johns Hopkins University, Baltimore MD. 3 Lujan Neutron Scattering Center, Los Alamos Neutron Science Center, Los Alamos National Laboratory, Los Alamos, NM. 4 Condensed Matter Physics and Materials Science Department, Brookhaven National Laboratory, Upton, NY. †[email protected] *[email protected] Based on specific heat and magnetoresistance measurements, we report that a “heavy” electronic state exists below T ≈ 20 K in KNi2Se2, with an increased carrier mobility and enhanced effective electronic band mass, m* = 6mb to 18mb. This “heavy” state evolves into superconductivity at Tc = 0.80(1) K. These properties resemble that of a many-body heavy-fermion state, which derives from the hybridization between localized magnetic states and conduction electrons. Yet, no evidence for localized magnetism or magnetic order is found in KNi2Se2 from magnetization measurements or neutron diffraction. Instead, neutron pair-distribution-function analysis reveals the presence of local charge-density-wave distortions that disappear on cooling, an effect opposite to what is typically observed, suggesting that the low-temperature electronic state of KNi2Se2 arises from cooperative Coulomb interactions and proximity to, but avoidance of, charge order. Introduction Methods KNi Se was synthesized from the elements as a Many-body states, in which complex phenomena 2 2 polycrystalline, lustrous, purple-red powder, as previously arise from simple interactions, often exhibit rich phase described12; the same batch of sample was used for all diagrams and lack formal predictive theories. -

Phonons – Thermal Properties
Phonons – Thermal properties Till now everything was classical – no quantization, no uncertainty.... WHY DO WE NEED QUANTUM THEORY OF LATTICE VIBRATIONS? Classical – Dulong Petit law All energy values are allowed – classical equipartition theorem – energy per degree of freedom is 1/2kBT. Total energy independent of temperature! Detailed calculation gives C = 3NkB In reality C ~ aT +bT3 Figure 1: Heat capacity of gold Need quantum theory! Quantum theory of lattice vibrations: Hamiltonian for 1D linear chain Interaction energy is simple harmonic type ~ ½ kx2 Solve it for N coupled harmonic oscillators – 3N normal modes – each with a characteristic frequency where p is the polarization/branch. Energy of any given mode is is the zero-point energy th is called ‘excitation number’ of the particular mode of p branch with wavevector k PH-208 Phonons – Thermal properties Page 1 Phonons are quanta of ionic displacement field that describes classical sound wave. Analogy th with black body radiation - ni is the number of photons of the i mode of oscillations of the EM wave. Total energy of the system E = is the ‘Planck distribution’ of the occupation number. Planck distribution: Consider an ensemble of identical oscillators at temperature T in thermal equilibrium. Ratio of number of oscillators in (n+1)th state to the number of them in nth state is So, the average occupation number <n> is: Special case of Bose-Einstein distribution with =0, chemical potential is zero since we do not directly control the total number of phonons (unlike the number of helium atoms is a bath – BE distribution or electrons in a solid –FE distribution), it is determined by the temperature. -

Experimental Determination of Heat Capacities and Their Correlation with Theoretical Predictions Waqas Mahmood, Muhammad Sabieh Anwar, and Wasif Zia
Experimental determination of heat capacities and their correlation with theoretical predictions Waqas Mahmood, Muhammad Sabieh Anwar, and Wasif Zia Citation: Am. J. Phys. 79, 1099 (2011); doi: 10.1119/1.3625869 View online: http://dx.doi.org/10.1119/1.3625869 View Table of Contents: http://ajp.aapt.org/resource/1/AJPIAS/v79/i11 Published by the American Association of Physics Teachers Additional information on Am. J. Phys. Journal Homepage: http://ajp.aapt.org/ Journal Information: http://ajp.aapt.org/about/about_the_journal Top downloads: http://ajp.aapt.org/most_downloaded Information for Authors: http://ajp.dickinson.edu/Contributors/contGenInfo.html Downloaded 13 Aug 2013 to 157.92.4.6. Redistribution subject to AAPT license or copyright; see http://ajp.aapt.org/authors/copyright_permission Experimental determination of heat capacities and their correlation with theoretical predictions Waqas Mahmood, Muhammad Sabieh Anwar,a) and Wasif Zia School of Science and Engineering, Lahore University of Management Sciences (LUMS), Opposite Sector U, D. H. A., Lahore 54792, Pakistan (Received 25 January 2011; accepted 23 July 2011) We discuss an experiment for determining the heat capacities of various solids based on a calorimetric approach where the solid vaporizes a measurable mass of liquid nitrogen. We demonstrate our technique for copper and aluminum, and compare our data with Einstein’s model of independent harmonic oscillators and the more accurate Debye model. We also illustrate an interesting material property, the Verwey transition