Species Status Assessment for the Reticulated Flatwoods Salamander (Ambystoma Bishopi) Version 1.0
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Zoology SYLLABUS 2016
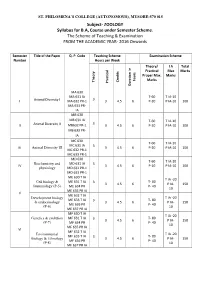
ST. PHILOMENA’S COLLEGE (AUTONOMOUS), MYSORE-570 015 Subject- ZOOLOGY Syllabus for B.A, Course under Semester Scheme. The Scheme of Teaching & Examination FROM THE ACADEMIC YEAR- 2016 Onwards Semester Title of the Paper Q. P. Code Teaching Scheme Examination Scheme Number Hours per Week Theory/ I A Total Practical Max Marks Proper Max. Marks hours Marks Theory Credits Practical Duration in in Duration MA 630 MA 631 IA T-60 T IA-10 3 I Animal Diversity I MA 632 PR-1 3 4.5 6 P-20 P IA-10 100 MA 633 PR- IA MB 630 MB 631 IA T-60 T IA-10 3 II Animal Diversity II MB632 PR-1 3 4.5 6 P-20 P IA-10 100 MB 633 PR- IA MC 630 T-60 T IA-10 MC 631 IA 3 III Animal Diversity III 3 4.5 6 P-20 P IA-10 100 MC 632 PR-1 MC 633 PR-1 MD 630 T-60 T IA-10 Biochemistry and MD 631 IA 3 IV 3 4.5 6 P-20 P IA-10 100 physiology MD 632 PR-1 MD 633 PR-1 ME 630 T IA T IA -20 Cell biology & ME 631 T IA 3 T- 80 3 4.5 6 P IA- 150 Immunology (P-5) ME 634 PR P- 40 10 ME 635 PR IA V ME 632 T IA Development biology T IA -20 ME 633 T IA 3 T- 80 & endocrinology 3 4.5 6 P IA- 150 ME 636 PR P- 40 (P-6) 10 ME 637 PR IA MF 630 T IA T IA -20 Genetics & evolution MF 631 T IA 3 T- 80 3 4.5 6 P IA- 150 (P-7) MF 634 PR P- 40 10 MF 635 PR IA VI MF 632 T IA Environmental T IA -20 MF 633 T IA 3 T- 80 Biology & Ethnology 3 4.5 6 P IA- 150 MF 636 PR P- 40 (P-8) 10 MF 637 PR IA ST. -

Louisiana Certified Habitat Plant List Native Woody Plants (Trees
Louisiana Certified Habitat Plant List Native Woody Plants (trees, shrubs, woody vines) Common name Scientific name Stewartia Gum, Swamp Black Nyssa biflora Camellia, Silky malacodendron Acacia, Sweet Acacia farnesiana Catalpa Gum, Tupelo Nyssa aquatica Liquidambar Alder, Black/Hazel Alnus rugosa Catalpa, Southern bignonioides Gum, Sweet styriciflua Allspice, Carolina/ Cedar, Eastern Red Juniperus virginiana Sweet Shrub Calycanthus floridus Cedar, Hackberry Celtis laevigata Ashes, Native Fraxinus spp. Atlantic/Southern Chamaecyparis Hawthorn, Native Crataegus spp. White thyoides Hawthorn, Barberry- Ash, Green F. pennsylvanicum Cherry, Black Prunus serotina leaf C. berberifolia Ash, Carolina F. caroliniana Hawthorn, Cherry, Choke Aronia arbutifolia Ash, Pumpkin F. profunda Blueberry C. brachycantha Cherry-laurel Prunus caroliniana Hawthorn, Green C. viridis Ash, White F. americana Chinquapin Castanea pumila Hawthorn, Mayhaw C. aestivalis/opaca Rhododendron Coralbean, Azalea, Pink canescens Eastern/Mamou Erythrina herbacea Hawthorn, Parsley C. marshallii Azalea, Florida Rhododendron Crabapple, Southern Malus angustifolia Hickories, Native Carya spp. Flame austrinum Creeper, Trumpet Campsis radicans Hickory, Black C. texana Anise, Star Illicium floridanum Parthenocissus Anise, Hickory, Bitternut C. cordiformes Creeper, Virginia quinquefolia Yellow/Florida Illicium parviflorum Hickory, Mockernut C. tomentosa Azalea, Florida Rhododendron Crossvine Bignonia capreolata Flame austrinum Hickory, Nutmeg C. myristiciformes Cucumber Tree Magnolia acuminata Rhododendron Hickory, PECAN C. illinoensis Azalea, Pink canescens Cypress, Bald Taxodium distichum Hickory, Pignut C. glabra Rhododendron Cypress, Pond Taxodium ascendens serrulatum, Hickory, Shagbark C. ovata Cyrilla, Swamp/Titi Cyrilla racemiflora viscosum, Hickory, Azalea, White oblongifolium Cyrilla, Little-leaf Cyrilla parvifolia Water/Bitter Pecan C. aquatica Baccharis/ Groundsel Bush Baccharis halimifolia Devil’s Walkingstick Aralia spinosa Hollies, Native Ilex spp. Baccharis, Salt- Osmanthus Holly, American I. -

Biosphere Consulting 14908 Tilden Road ‐ Winter Garden FL 34787 (407) 656 8277
Biosphere Consulting 14908 Tilden Road ‐ Winter Garden FL 34787 (407) 656 8277 www.BiosphereNursery.com The following list of plants include only native wetland and transitional species used primarily in aquascaping, lakefront and wetland restoration. Biosphere also carries a large number of upland species and BIOSCAPE species, as well as wildflower seeds and plants. The nursery is open to the public on Tuesday through Saturday only from 9:00 A.M. until 5:00 P.M. Prices are F.O.B. the nursery. * Bare root plants must be ordered at least two (2) days prior to pick-up. PRICE LIST NATIVE WETLAND AND TRANSITIONAL SPECIES HERBACEOUS SPECIES *Bare Root 1 Gal. 3 Gal. Arrowhead (Sagittaria latifolia) .50 2.50 --- Bulrush (Scirpus californicus & S.validus) .50 --- 8.00 Burrmarigold (Bidens leavis) --- 2.00 --- Canna (Canna flaccida) .60 2.00 --- Crinum (Crinum americanum) 1.50 3.00 10.00 Duck Potato (Sagittaria lancifolia) .60 2.00 --- Fragrant Water Lily (Nymphaea odorata) 5.00 --- 12.00 Hibiscus (Hibiscus coccinea) --- 3.00 8.00 Horsetail (Equisetum sp.) .80 2.00 --- Iris (Iris savannarum) .60 2.00 --- Knotgrass (Paspalum distichum) .50 2.00 --- Lemon Bacopa (Bacopa caroliniana) --- 3.50 --- Lizards Tail (Saururus cernuus) .60 2.50 --- Maidencane (Panicum hemitomon) .50 2.00 --- Pickerelweed (Pontederia cordata) .50 2.00 --- Redroot (Lachnanthes carolinana) .60 2.00 --- Sand Cord Grass (Spartina bakeri) .50 3.50 --- Sawgrass (Cladium jamaicense) .60 3.00 --- Softrush (Juncus effusus) .50 2.00 --- Spikerush (Eleocharis cellulosa) .70 2.00 --- -

Revision of the Eurycea Quadridigitata
Revision of the Eurycea quadridigitata (Holbrook 1842) Complex of Dwarf Salamanders (Caudata: Plethodontidae: Hemidactyliinae) with a Description of Two New Species Author(s): Kenneth P. Wray, D. Bruce Means, and Scott J. Steppan Source: Herpetological Monographs, 31(1):18-46. Published By: The Herpetologists' League DOI: http://dx.doi.org/10.1655/HERPMONOGRAPHS-D-16-00011 URL: http://www.bioone.org/doi/full/10.1655/HERPMONOGRAPHS-D-16-00011 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Herpetological Monographs, 31, 2017, 18–46 Ó 2017 by The Herpetologists’ League, Inc. Revision of the Eurycea quadridigitata (Holbrook 1842) Complex of Dwarf Salamanders (Caudata: Plethodontidae: Hemidactyliinae) with a Description of Two New Species 1,3 1,2 1 KENNETH P. WRAY ,D.BRUCE MEANS , AND SCOTT J. STEPPAN 1 Department of Biological Science, Florida State University, Tallahassee, FL 32306-4295, USA 2 Coastal Plains Institute and Land Conservancy, 1313 Milton Street, Tallahassee, FL 32303-5512, USA ABSTRACT: The Eurycea quadridigitata complex is currently composed of the nominate species and E. -

Amphibiaweb's Illustrated Amphibians of the Earth
AmphibiaWeb's Illustrated Amphibians of the Earth Created and Illustrated by the 2020-2021 AmphibiaWeb URAP Team: Alice Drozd, Arjun Mehta, Ash Reining, Kira Wiesinger, and Ann T. Chang This introduction to amphibians was written by University of California, Berkeley AmphibiaWeb Undergraduate Research Apprentices for people who love amphibians. Thank you to the many AmphibiaWeb apprentices over the last 21 years for their efforts. Edited by members of the AmphibiaWeb Steering Committee CC BY-NC-SA 2 Dedicated in loving memory of David B. Wake Founding Director of AmphibiaWeb (8 June 1936 - 29 April 2021) Dave Wake was a dedicated amphibian biologist who mentored and educated countless people. With the launch of AmphibiaWeb in 2000, Dave sought to bring the conservation science and basic fact-based biology of all amphibians to a single place where everyone could access the information freely. Until his last day, David remained a tirelessly dedicated scientist and ally of the amphibians of the world. 3 Table of Contents What are Amphibians? Their Characteristics ...................................................................................... 7 Orders of Amphibians.................................................................................... 7 Where are Amphibians? Where are Amphibians? ............................................................................... 9 What are Bioregions? ..................................................................................10 Conservation of Amphibians Why Save Amphibians? ............................................................................. -

Department of the Interior Fish and Wildlife Service
Tuesday, February 10, 2009 Part II Department of the Interior Fish and Wildlife Service 50 CFR Part 17 Endangered and Threatened Wildlife and Plants; Determination of Endangered Status for Reticulated Flatwoods Salamander; Designation of Critical Habitat for Frosted Flatwoods Salamander and Reticulated Flatwoods Salamander; Final Rule VerDate Nov<24>2008 14:17 Feb 09, 2009 Jkt 217001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\10FER2.SGM 10FER2 erowe on PROD1PC63 with RULES_2 6700 Federal Register / Vol. 74, No. 26 / Tuesday, February 10, 2009 / Rules and Regulations DEPARTMENT OF THE INTERIOR during normal business hours, at U.S. Register on or before July 30, 2008, with Fish and Wildlife Service, Mississippi the final critical habitat rule to be Fish and Wildlife Service Fish and Wildlife Office, 6578 Dogwood submitted for publication in the Federal View Parkway, Jackson, MS 39213. Register by January 30, 2009. The 50 CFR Part 17 FOR FURTHER INFORMATION CONTACT: Ray revised proposed rule was signed on [FWS–R4–ES–2008–0082; MO 9921050083– Aycock, Field Supervisor, U.S. Fish and and delivered to the Federal Register on B2] Wildlife Service, Mississippi Field July 30, 2008, and it subsequently Office, 6578 Dogwood View Parkway, published on August 13, 2008 (73 FR RIN 1018–AU85 Jackson, MS 39213; telephone: 601– 47258). We also published supplemental information on the Endangered and Threatened Wildlife 321–1122; facsimile: 601–965–4340. If proposed rule to maintain the status of and Plants; Determination of you use a telecommunications device the frosted flatwoods salamander as Endangered Status for Reticulated for the deaf (TDD), call the Federal Information Relay Service (FIRS) at threatened (73 FR 54125; September 18, Flatwoods Salamander; Designation of 2008). -

Successful Reproduction of the Mole Salamander Ambystoma Talpoideum in Captivity, with an Emphasis on Stimuli Environmental Determinants
SHORT NOTE The Herpetological Bulletin 141, 2017: 28-31 Successful reproduction of the mole salamander Ambystoma talpoideum in captivity, with an emphasis on stimuli environmental determinants AXEL HERNANDEZ Department of Environmental Sciences, Faculty of Sciences and Technics, University Pasquale Paoli of Corsica, Corte, 20250, France Author Email: [email protected] ABSTRACT - Generating and promoting evidence-based husbandry protocols for urodeles, commonly known as newts and salamanders, is urgently needed because most of the up-to-date ex situ programs are focused on frogs and toads than Urodela. Data on biology, life history, ecology and environmental parameters are lacking for many species and are needed to establish suitable husbandry and breeding conditions in captive environments. Two adult females and two adult males, of the mole salamander Ambystoma talpoideum successfully reproduced in captivity. It was found that reproduction of this species depends on various complex stimuli: including natural photoperiod 12:12, rainwater (acidic to neutral pH) and an aquarium full of various debris. Additionally high temperature variations ranging from 2 °C to 17 °C (a decrease followed by an increase) between November and February showed that it is possible to breed adults in aquariums provided the right stimuli are applied at the right moment of time in winter. A. talpoideum shows an explosive breeding mode as previously reported for the whole genus Ambystoma. INTRODUCTION with an emphasis on the environmental determinant stimuli involved. These data may assist in improving breeding these ince the 1980s, the current global amphibian extinction salamanders under artificial conditions. crisis has been discussed and acknowledged (Wake, A. -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

ISB: Atlas of Florida Vascular Plants
Longleaf Pine Preserve Plant List Acanthaceae Asteraceae Wild Petunia Ruellia caroliniensis White Aster Aster sp. Saltbush Baccharis halimifolia Adoxaceae Begger-ticks Bidens mitis Walter's Viburnum Viburnum obovatum Deer Tongue Carphephorus paniculatus Pineland Daisy Chaptalia tomentosa Alismataceae Goldenaster Chrysopsis gossypina Duck Potato Sagittaria latifolia Cow Thistle Cirsium horridulum Tickseed Coreopsis leavenworthii Altingiaceae Elephant's foot Elephantopus elatus Sweetgum Liquidambar styraciflua Oakleaf Fleabane Erigeron foliosus var. foliosus Fleabane Erigeron sp. Amaryllidaceae Prairie Fleabane Erigeron strigosus Simpson's rain lily Zephyranthes simpsonii Fleabane Erigeron vernus Dog Fennel Eupatorium capillifolium Anacardiaceae Dog Fennel Eupatorium compositifolium Winged Sumac Rhus copallinum Dog Fennel Eupatorium spp. Poison Ivy Toxicodendron radicans Slender Flattop Goldenrod Euthamia caroliniana Flat-topped goldenrod Euthamia minor Annonaceae Cudweed Gamochaeta antillana Flag Pawpaw Asimina obovata Sneezeweed Helenium pinnatifidum Dwarf Pawpaw Asimina pygmea Blazing Star Liatris sp. Pawpaw Asimina reticulata Roserush Lygodesmia aphylla Rugel's pawpaw Deeringothamnus rugelii Hempweed Mikania cordifolia White Topped Aster Oclemena reticulata Apiaceae Goldenaster Pityopsis graminifolia Button Rattlesnake Master Eryngium yuccifolium Rosy Camphorweed Pluchea rosea Dollarweed Hydrocotyle sp. Pluchea Pluchea spp. Mock Bishopweed Ptilimnium capillaceum Rabbit Tobacco Pseudognaphalium obtusifolium Blackroot Pterocaulon virgatum -

Florida Bog Frog Rana Okaloosae Taxa: Amphibian SE-GAP Spp Code: Afbfr Order: Anura ITIS Species Code: 173456 Family: Ranidae Natureserve Element Code: AAABH01240
Florida Bog Frog Rana okaloosae Taxa: Amphibian SE-GAP Spp Code: aFBFR Order: Anura ITIS Species Code: 173456 Family: Ranidae NatureServe Element Code: AAABH01240 KNOWN RANGE: PREDICTED HABITAT: P:\Proj1\SEGap P:\Proj1\SEGap Range Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Range_aFBFR.pdf Predicted Habitat Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Dist_aFBFR.pdf GAP Online Tool Link: http://www.gapserve.ncsu.edu/segap/segap/index2.php?species=aFBFR Data Download: http://www.basic.ncsu.edu/segap/datazip/region/vert/aFBFR_se00.zip PROTECTION STATUS: Reported on March 14, 2011 Federal Status: --- State Status: FL (SSC) NS Global Rank: G2 NS State Rank: FL (S2) aFBFR Page 1 of 3 SUMMARY OF PREDICTED HABITAT BY MANAGMENT AND GAP PROTECTION STATUS: US FWS US Forest Service Tenn. Valley Author. US DOD/ACOE ha % ha % ha % ha % Status 1 0.0 0 0.0 0 0.0 0 0.0 0 Status 2 0.0 0 0.0 0 0.0 0 0.0 0 Status 3 0.0 0 0.0 0 0.0 0 7,002.6 29 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 0.0 0 0.0 0 0.0 0 7,002.6 29 US Dept. of Energy US Nat. Park Service NOAA Other Federal Lands ha % ha % ha % ha % Status 1 0.0 0 0.0 0 0.0 0 0.0 0 Status 2 0.0 0 1.1 < 1 0.0 0 0.0 0 Status 3 0.0 0 0.0 0 0.0 0 0.0 0 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 0.0 0 1.1 < 1 0.0 0 0.0 0 Native Am. -

The Black~-Banded Sunfish ~ Enneacanthu~ Chaetodon
THE BLACK~-BANDED SUNFISH ~ ENNEACANTHU~ CHAETODON The Blac~-banded Sunfish was one of the first native fishes to be kept by American aquartsts, Shimmering silver and black, gliding majestically through the aquascape, they present an exciting cha llenge to the keeper of indigenous fishes. To encourage fellow aquar.~3tS to acquire and breed this miniature beauty We will att empt to review past literature and relate it to our own·observa tions. The Black-banded Sunfish belongs to the order of "perch-shaped :f5_sh~':s" or Perciformes, sub-order Percoidei, and the family Cen tx·archidae,Named Pomotis chaetodon by Baird in .L854, it was later renamed Mesogonist~us chaetodor and is currently known as Ennea canthus chaetodon. We affectionately call them "chaets" ("keets") to s~mpl~fy th~ngs. · Stoye (1971) notes that E. chaetodon (chaets) were first collect- .ed in the.swamps of Southern New Jersey and introduced into Ger many in 1897, although they were not kept by American aquarists until 1910. In addition to New Jersey the range includes Maryland, Delaware, Virginia, North Carolina, and Florida. Informative reports by Quinn {1967a,b) and Coombs(l97J) recorded water acidity at 6,4 pH and below in some areas. Quinn observes that "waving fronds of sphagnum moss" and decaying plant material are abundant in the lowlands of the pine barrensa these factors account, for the most part, for the acidic water quality. Although we have ·acclimated chaets to 8,2 pH water, we found that they stayed in better health and bred when maintained at 7,0 pH or be low. -

AMPHIBIANS of OHIO F I E L D G U I D E DIVISION of WILDLIFE INTRODUCTION
AMPHIBIANS OF OHIO f i e l d g u i d e DIVISION OF WILDLIFE INTRODUCTION Amphibians are typically shy, secre- Unlike reptiles, their skin is not scaly. Amphibian eggs must remain moist if tive animals. While a few amphibians Nor do they have claws on their toes. they are to hatch. The eggs do not have are relatively large, most are small, deli- Most amphibians prefer to come out at shells but rather are covered with a jelly- cately attractive, and brightly colored. night. like substance. Amphibians lay eggs sin- That some of these more vulnerable spe- gly, in masses, or in strings in the water The young undergo what is known cies survive at all is cause for wonder. or in some other moist place. as metamorphosis. They pass through Nearly 200 million years ago, amphib- a larval, usually aquatic, stage before As with all Ohio wildlife, the only ians were the first creatures to emerge drastically changing form and becoming real threat to their continued existence from the seas to begin life on land. The adults. is habitat degradation and destruction. term amphibian comes from the Greek Only by conserving suitable habitat to- Ohio is fortunate in having many spe- amphi, which means dual, and bios, day will we enable future generations to cies of amphibians. Although generally meaning life. While it is true that many study and enjoy Ohio’s amphibians. inconspicuous most of the year, during amphibians live a double life — spend- the breeding season, especially follow- ing part of their lives in water and the ing a warm, early spring rain, amphib- rest on land — some never go into the ians appear in great numbers seemingly water and others never leave it.