Herpetofaunal Communities in Ephemeral Wetlands Embedded Within Longleaf Pine Flatwoods of the Gulf Coastal Plain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ornate Chorus Frog
Ornate Chorus Frog Pseudacris ornata Taxa: Amphibian SE-GAP Spp Code: aOCFR Order: Anura ITIS Species Code: 173531 Family: Hylidae NatureServe Element Code: AAABC05050 KNOWN RANGE: PREDICTED HABITAT: P:\Proj1\SEGap P:\Proj1\SEGap Range Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Range_aOCFR.pdf Predicted Habitat Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Dist_aOCFR.pdf GAP Online Tool Link: http://www.gapserve.ncsu.edu/segap/segap/index2.php?species=aOCFR Data Download: http://www.basic.ncsu.edu/segap/datazip/region/vert/aOCFR_se00.zip PROTECTION STATUS: Reported on March 14, 2011 Federal Status: --- State Status: MS (Non-game species in need of management), NC (SR) NS Global Rank: G5 NS State Rank: AL (S5), FL (SNR), GA (S5), LA (S1), MS (S1), NC (S3), SC (SNR) aOCFR Page 1 of 4 SUMMARY OF PREDICTED HABITAT BY MANAGMENT AND GAP PROTECTION STATUS: US FWS US Forest Service Tenn. Valley Author. US DOD/ACOE ha % ha % ha % ha % Status 1 14,156.0 < 1 2,389.3 < 1 0.0 0 0.0 0 Status 2 32,041.4 < 1 24,919.3 < 1 0.0 0 0.0 0 Status 3 3.2 < 1 181,436.9 4 0.0 0 75,398.1 2 Status 4 5.2 < 1 0.0 0 0.0 0 0.0 0 Total 46,205.9 < 1 208,745.5 4 0.0 0 75,398.1 2 US Dept. of Energy US Nat. Park Service NOAA Other Federal Lands ha % ha % ha % ha % Status 1 0.0 0 7,947.2 < 1 8.6 < 1 706.9 < 1 Status 2 0.0 0 1,356.0 < 1 1,336.1 < 1 0.0 0 Status 3 13,565.2 < 1 84.2 < 1 0.0 0 1,051.8 < 1 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 13,565.2 < 1 9,387.5 < 1 1,344.6 < 1 1,758.7 < 1 Native Am. -

Revision of the Eurycea Quadridigitata
Revision of the Eurycea quadridigitata (Holbrook 1842) Complex of Dwarf Salamanders (Caudata: Plethodontidae: Hemidactyliinae) with a Description of Two New Species Author(s): Kenneth P. Wray, D. Bruce Means, and Scott J. Steppan Source: Herpetological Monographs, 31(1):18-46. Published By: The Herpetologists' League DOI: http://dx.doi.org/10.1655/HERPMONOGRAPHS-D-16-00011 URL: http://www.bioone.org/doi/full/10.1655/HERPMONOGRAPHS-D-16-00011 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Herpetological Monographs, 31, 2017, 18–46 Ó 2017 by The Herpetologists’ League, Inc. Revision of the Eurycea quadridigitata (Holbrook 1842) Complex of Dwarf Salamanders (Caudata: Plethodontidae: Hemidactyliinae) with a Description of Two New Species 1,3 1,2 1 KENNETH P. WRAY ,D.BRUCE MEANS , AND SCOTT J. STEPPAN 1 Department of Biological Science, Florida State University, Tallahassee, FL 32306-4295, USA 2 Coastal Plains Institute and Land Conservancy, 1313 Milton Street, Tallahassee, FL 32303-5512, USA ABSTRACT: The Eurycea quadridigitata complex is currently composed of the nominate species and E. -

Title Freshwater Fishes, Terrestrial Herpetofauna and Mammals of Pulau Tekong, Singapore Author(S) Kelvin K.P
Title Freshwater fishes, terrestrial herpetofauna and mammals of Pulau Tekong, Singapore Author(s) Kelvin K.P. Lim, Marcus A. H., Chua and Norman T-L. Lim Source Nature in Singapore, 9, 165–198 Published by Lee Kong Chian Natural History Museum, National University of Singapore Copyright © 2016 National University of Singapore This document may be used for private study or research purpose only. This document or any part of it may not be duplicated and/or distributed without permission of the copyright owner. The Singapore Copyright Act applies to the use of this document. This document first appeared in: Lim, K. K. P., Chua, M. A. H., & Lim, N. T. -L. (2016). Freshwater fishes, terrestrial herpetofauna and mammals of Pulau Tekong, Singapore. Nature in Singapore, 9, 165–198. Retrieved from http://lkcnhm.nus.edu.sg/nus/images/pdfs/nis/2016/2016nis165-198.pdf This document was archived with permission from the copyright owner. NATURE IN SINGAPORE 2016 9: 165–198 Date of Publication: 1 November 2016 © National University of Singapore Freshwater fishes, terrestrial herpetofauna and mammals of Pulau Tekong, Singapore Kelvin K.P. Lim1*, Marcus A. H. Chua1 & Norman T-L. Lim2 1Lee Kong Chian Natural History Museum, National University of Singapore, Singapore 117377, Republic of Singapore; Email: [email protected] (KKPL; *corresponding author), [email protected] (MAHC) 2Natural Sciences and Science Education Academic Group, National Institute of Education, Nanyang Technological University, 1 Nanyang Walk, Singapore 637616, Republic of Singapore; Email: [email protected] (NTLL) Abstract. The diversity of terrestrial and freshwater, non-avian, vertebrate fauna of Pulau Tekong, an island used almost exclusively by the Singapore Armed Forces, was compiled. -

Eight Novel Tetranucleotide and Five Cross-Species Dinucleotide Microsatellite Loci for the Ornate Chorus Frog (Pseudacris Ornata)
Molecular Ecology Resources (2009) 9, 622–624 doi: 10.1111/j.1755-0998.2008.02478.x PERMANENTBlackwell Publishing Ltd GENETIC RESOURCES NOTE Eight novel tetranucleotide and five cross-species dinucleotide microsatellite loci for the ornate chorus frog (Pseudacris ornata) JACOB F. DEGNER,* TYLER D. HETHER and ERIC A. HOFFMAN Department of Biology, University of Central Florida, 4000 Central Florida Blvd., Orlando, Florida 32816, USA Abstract We describe the cloning and characterization of eight novel tetranucleotide microsatellite loci in the ornate chorus frog (Pseudacris ornata). We also screened 26 loci from GenBank that were isolated from other Pseudacris species and obtained consistent product from five of these dinucleotide loci. All loci are polymorphic. In our sample of 26 frogs from a natural population, polymorphism ranged from 1 to 22 alleles per locus with expected heterozygosities ranging from 0 to 0.958. These loci enable high-resolution studies of P. ornata. Moreover, cross-species amplification success suggests they will also be useful for other chorus frog species. Keywords: biotin enrichment, chorus frog, microsatellite, Pseudacris ornata Received 22 July 2008; revision accepted 15 October 2008 The ornate chorus frog (Pseudacris ornata) is a small terrestrial motif bound to streptavidin-coated particles (Promega) frog occurring in the coastal plains of the southeastern enriched via magnetic separation. Enriched product was USA. Moriarty & Cannatella (2004) suggest some degree of cloned using TOPO TA cloning kits (Invitrogen). PCR phylogeographical structure in this species although gene screening followed the procedure from Cabe & Marshal flow and high-resolution population genetic structure has (2001). Briefly, two PCRs were carried out per sample. -

2017 Annual Report the Wildlife Center of Virginia … a Hospital for Native Wildlife
The Wildlife Center of Virginia 2017 Annual Report The Wildlife Center of Virginia … a hospital for native wildlife. During 2017, the Wildlife Center admitted 2,768 patients – sick, injured, and orphaned wild animals from all across Virginia, and our highest caseload since 2002. Among the 2017 patients were: ■ 403 Eastern Cottontails; ■ 10 different species of bats; ■ 329 Virginia Opossums; ■ One Fowler’s Toad (cover photo). This adult toad was accidentally stepped on and suffered a dislocated left ■ 198 Eastern Gray Squirrels; femur. After four weeks of physical therapy, the toad ■ 119 Eastern Box Turtles; was returned to the wild. A record-setting 55 Bald Eagles, shattering the previous record of 42 eagles set in 2012. About 70 percent of these 55 eagles came in with detectable levels of lead in their blood. Eagles are ingesting fragments of lead bullets and shotgun pellets left in the remains of deer and small game that have been shot with lead-based ammunition. A lead fragment no bigger than a small grain of rice can kill a Bald Eagle. There are treatment options available – if the eagle is treated in time. The Wildlife Center is also leading a national campaign to encourage hunters to switch to non- lead ammunition. 2 2017 Patient Admissions Total Admissions 2,768 Northern Diamondback Terrapin 2 Horned Lark 1 Northern Ring-necked Snake 2 House Finch 36 Mammals [1,306 patients] Northern Rough Greensnake 1 House Sparrow 18 American Beaver 3 Red Cornsnake 2 House Wren 4 American Black Bear 21 Red-eared Slider 3 Northern Cardinal 39 Big -

Divergent Influenza-Like Viruses of Amphibians and Fish Support
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.27.270959; this version posted August 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Divergent influenza-like viruses of amphibians and fish support 2 an ancient evolutionary association 3 4 5 Rhys Parry1, #, *, Michelle Wille2, #, Olivia M. H. Turnbull3, Jemma L. Geoghegan4,5, Edward C. 6 Holmes2,* 7 1 Australian Infectious Diseases Research Centre, School of Chemistry and Molecular Biosciences, 8 The University of Queensland, Brisbane, QLD 4067, Australia; [email protected] (R.P) 9 2 Marie Bashir Institute for Infectious Diseases and Biosecurity, School of Life & Environmental 10 Sciences and School of Medical Sciences, The University of Sydney, Sydney, NSW 2006, 11 Australia; [email protected] (M.W.); [email protected] (E.C.H) 12 3 Department of Biological Sciences, Macquarie University, Sydney, NSW 2109, Australia; 13 [email protected] (O.M.H.T) 14 4 Department of Microbiology and Immunology, University of Otago, Dunedin 9016, New Zealand; 15 [email protected] (J.L.G) 16 5 Institute of Environmental Science and Research, Wellington 5018, New Zealand 17 18 # These authors contributed equally 19 20 * Correspondence: [email protected] Tel +617 3365 3302 (R.P); [email protected] Tel 21 +61 2 9351 5591 (E.C.H) 22 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.27.270959; this version posted August 27, 2020. -

Chamberlain's Dwarf Salamander
Chamberlain's Dwarf Salamander Eurycea chamberlaini Taxa: Amphibian SE-GAP Spp Code: aCDSA Order: Caudata ITIS Species Code: --- Family: Plethodontidae NatureServe Element Code: AAAAD05092 KNOWN RANGE: PREDICTED HABITAT: P:\Proj1\SEGap P:\Proj1\SEGap Range Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Range_aCDSA.pdf Predicted Habitat Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Dist_aCDSA.pdf GAP Online Tool Link: http://www.gapserve.ncsu.edu/segap/segap/index2.php?species=aCDSA Data Download: http://www.basic.ncsu.edu/segap/datazip/region/vert/aCDSA_se00.zip PROTECTION STATUS: Reported on March 14, 2011 Federal Status: --- State Status: --- NS Global Rank: G5 NS State Rank: FL (SNR), GA (S1), NC (S3S4), SC (SNR) aCDSA Page 1 of 3 SUMMARY OF PREDICTED HABITAT BY MANAGMENT AND GAP PROTECTION STATUS: US FWS US Forest Service Tenn. Valley Author. US DOD/ACOE ha % ha % ha % ha % Status 1 0.0 0 0.0 0 0.0 0 0.0 0 Status 2 2,439.0 < 1 164.7 < 1 0.0 0 0.0 0 Status 3 0.0 0 6,240.3 1 0.0 0 6,576.0 1 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 2,439.0 < 1 6,405.0 1 0.0 0 6,576.0 1 US Dept. of Energy US Nat. Park Service NOAA Other Federal Lands ha % ha % ha % ha % Status 1 0.0 0 7,492.0 1 0.0 0 0.0 0 Status 2 0.0 0 0.0 0 0.0 0 0.0 0 Status 3 14,041.6 2 31.3 < 1 0.0 0 0.8 < 1 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 14,041.6 2 7,523.3 1 0.0 0 0.8 < 1 Native Am. -

Standard Common and Current Scientific Names for North American Amphibians, Turtles, Reptiles & Crocodilians
STANDARD COMMON AND CURRENT SCIENTIFIC NAMES FOR NORTH AMERICAN AMPHIBIANS, TURTLES, REPTILES & CROCODILIANS Sixth Edition Joseph T. Collins TraVis W. TAGGart The Center for North American Herpetology THE CEN T ER FOR NOR T H AMERI ca N HERPE T OLOGY www.cnah.org Joseph T. Collins, Director The Center for North American Herpetology 1502 Medinah Circle Lawrence, Kansas 66047 (785) 393-4757 Single copies of this publication are available gratis from The Center for North American Herpetology, 1502 Medinah Circle, Lawrence, Kansas 66047 USA; within the United States and Canada, please send a self-addressed 7x10-inch manila envelope with sufficient U.S. first class postage affixed for four ounces. Individuals outside the United States and Canada should contact CNAH via email before requesting a copy. A list of previous editions of this title is printed on the inside back cover. THE CEN T ER FOR NOR T H AMERI ca N HERPE T OLOGY BO A RD OF DIRE ct ORS Joseph T. Collins Suzanne L. Collins Kansas Biological Survey The Center for The University of Kansas North American Herpetology 2021 Constant Avenue 1502 Medinah Circle Lawrence, Kansas 66047 Lawrence, Kansas 66047 Kelly J. Irwin James L. Knight Arkansas Game & Fish South Carolina Commission State Museum 915 East Sevier Street P. O. Box 100107 Benton, Arkansas 72015 Columbia, South Carolina 29202 Walter E. Meshaka, Jr. Robert Powell Section of Zoology Department of Biology State Museum of Pennsylvania Avila University 300 North Street 11901 Wornall Road Harrisburg, Pennsylvania 17120 Kansas City, Missouri 64145 Travis W. Taggart Sternberg Museum of Natural History Fort Hays State University 3000 Sternberg Drive Hays, Kansas 67601 Front cover images of an Eastern Collared Lizard (Crotaphytus collaris) and Cajun Chorus Frog (Pseudacris fouquettei) by Suzanne L. -

Proposed Amendment to 21CFR124021
Richard Fife 8195 S. Valley Vista Drive Hereford, AZ 85615 December 07, 2015 Division of Dockets Management Food and Drug Administration 5630 Fishers Lane, rm. 1061 Rockville, MD 20852 Reference: Docket Number FDA-2013-S-0610 Proposed Amendment to Code of Federal Regulations Title 21, Volume 8 Revised as of April 1, 2015 21CFR Sec.1240.62 Dear Dr. Stephen Ostroff, M.D., Acting Commissioner: Per discussion with the Division of Dockets Management staff on November 10, 2015 Environmental and Economic impact statements are not required for petitions submitted under 21CFR Sec.1240.62 CITIZEN PETITION December 07, 2015 ACTION REQUESTED: I propose an amendment to 21CFR Sec.1240.62 (see exhibit 1) as allowed by Section (d) Petitions as follows: Amend section (c) Exceptions. The provisions of this section are not applicable to: By adding the following two (2) exceptions: (5) The sale, holding for sale, and distribution of live turtles and viable turtle eggs, which are sold for a retail value of $75 or more (not to include any additional turtle related apparatuses, supplies, cages, food, or other turtle related paraphernalia). This dollar amount should be reviewed every 5 years or more often, as deemed necessary by the department in order to make adjustments for inflation using the US Department of Labor, Bureau of labor Statistics, Consumer Price Index. (6) The sale, holding for sale, and distribution of live turtles and viable turtle eggs, which are listed by the International Union for Conservation of Nature and Natural Resources (IUCN) Red List as Extinct In Wild, Critically Endangered, Endangered, or Vulnerable (IUCN threatened categorizes). -

Danh Lục Ếch Nhái
Danh lục Ếch nhái - Vườn Quốc Gia Cát Tiên version: 28 January 2021 List of Amphibians in Cát Tiên National Park Order Family VN En Notes Gymnophiona Họ Ếch giun Asiatic tailed caecilians or fish caecilians Ichthyophiidae Ichthyophis catlocensis Ếch giun Cát Lộc Cát Lộc caecilia endemic Ichthyophis nguyenorum Ếch giun Nguyễn Nguyen's caecilia Anura Megophryidae Họ Cóc bùn "goose frogs" Brachytarsophrys intermedia Cóc mày trung gian Annam spadefoot toad VU * Megophrys major Ếch sừng châu Á Asian horned frog (genus) Ophryophryne synoria Bufonidae Họ Cóc Typical Toads Duttaphrynus melanostictus Cóc nhà common Asian toad was Bufo Ingerophrynus (= Bufo) galeatus Cóc rừng bony-headed toad; forest toad Microhylidae Họ Nhái Bầu Narrow-mouthed frogs Glyphoglossus guttulatus Ễnh ương đốm blotched burrowing frog (synonym Calluella guttulata) Glyphoglossus molossus Nhái lưỡi Broad-lipped frog, balloon frog * Kalophrynus cryptophonus Cóc đốm tre Kalophrynus interlineatus Nhái cóc đốm Northern Sticky Frog or Snoring Frog Kaloula indochinensis Kaloula pulchra Ễnh ương thường Banded Bullfrog or Asian Painted Frog Microhyla berdmorei Nhái bầu béc mơ Berdmore's Narrow-Mouthed Frog Microhyla butleri Nhái bầu bút lơ Butler's Pigmy Frog Microhyla fissipes ornate chorus frog Microhyla heymonsi Nhái bầu Hây môn Black-flanked Pigmy Frog Microhyla inornata Nhái bầu trơn Jewel Pigmy Frog ¶ Microhyla ornata Nhái bầu hoa Ornate Pigmy Frog ¶ Microhyla pulchra Nhái bầu vân Woodgrain Pigmy Frog, marbled pygmy frog Micryletta erythropoda Nhái bầu chân đỏ Mada paddy -

Field Journal.Pub
The reserve has thousands of acres of wildlands that sup- port hundreds of species of plants and animals. Although most of the plants and animals are harmless, there are some that can cause harm if they bite, sting or rub up Grand Bay against you. As long as you exercise caution, you should have no problem with them. Many of these harmful inter- National Estuarine actions (except with the bugs) can be avoided if you stay out of the water and on the trails. Remember! The number Research Reserve one person responsible for your safety is YOU! Plants to avoid contact with: Field Journal Poison Ivy Remember! Leaflets three—leave it be. Black Needlerush Don’t let it poke you in the eye. Animals to avoid contact with: Alligators Poisonous Snakes Jellyfish Stingrays Sharks Louisiana Black Bears (rare) Biting Bugs including: Ticks, Deer Flies, Mosquitoes and Gnats Please Do Not Feed the Wildlife! 6005 Bayou Heron Road, Moss Point, MS 39562 Pone: 228.475.7047 18 Field Notes and Drawings Grand Bay NERR and surrounding areas 17 2 Field Notes and Drawings A Visitor’s Field Journal Note to Users This field journal was compiled in order to pro- vide you, our visitors, with species lists of common ani- mals and plants found within the protected wildlands of the Grand Bay National Estuarine Research Reserve and Refuge. We encourage you to cautiously explore these sensitive areas on foot when you are out in the field. Many of our coastal habitats such as pine savan- nas and salt pannes are extremely fragile in nature. -
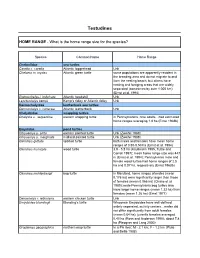
Home Range Testudines
Testudines HOME RANGE - What is the home range size for the species? Species Common Name Home Range Cheloniidae sea turtles Caretta c. caretta Atlantic loggerhead Unk Chelonia m. mydas Atlantic green turtle some populations are apparently resident in the breeding area and do not migrate to and from the nesting beach, but others have nesting and foraging areas that are widely separated (sometimes by over 1,000 km) (Ernst et al. 1994) Eretmochelys i. imbricata Atlantic hawksbill Unk Lepidochelys kempii Kemp’s ridley or Atlantic ridley Unk Dermochelyidae leatherback sea turtles Dermochelys c. coriacea Atlantic leatherback Unk Chelydridae snapping turtles Chelydra s. serpentina eastern snapping turtle in Pennsylvanina, nine adults…had estimated home ranges averaging 1.8 ha (Ernst 1968b) Emydidae pond turtles Chrysemys p. picta eastern painted turtle Unk (Zweifel 1989) Chrysemys p. marginata midland painted turtle Unk (Zweifel 1989) Clemmys guttata spotted turtle both males and females have mean home ranges of 0.50-0.53 ha (Ernst et al. 1994) Clemmys insculpta wood turtle 3.9 - 5.8 ha (Kaufmann 1995, Tuttle and Carroll 1997); mean home range size was 447 m (Ernst et al. 1994); Pennsylvania male and female wood turtles had home ranges of 2.5 ha and 0.07 ha, respectively (Ernst 1968b) Clemmys muhlenbergii bog turtle in Maryland, home ranges of males (mean 0.176 ha) were significantly larger than those of females (mean 0.066 ha) (Chase et al. 1989); male Pennsylvania bog turtles also have larger home ranges (mean 1.33 ha) than females (mean 1.26 ha) (Ernst 1977) Deirochelys r.