The Evolutionary Origin of “Black” Kokanee (Oncorhynchus Nerka) and Their
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Squamish-Lillooet Regional District Electoral Area C Official Community
Electoral Area C Official Community Plan Bylaw No. 689, 1999 CONSOLIDATED COPY March 2015 IMPORTANT NOTICE THIS IS AN UNOFFICIAL CONSOLIDATION OF BYLAW NO. 689 WHICH HAS BEEN PREPARED FOR CONVENIENCE ONLY. Although the Squamish-Lillooet Regional District is careful to assure the accuracy of all information presented in this consolidation, you should confirm all information before making any decisions based on it. Information can be confirmed through the SLRD Planning Department. Official Community Plan Bylaw 689 (Consolidated for Convenience Only) March 2015 SUMMARY OF AMENDMENTS CONSOLIDATED FOR CONVENIENCE ONLY Consolidated bylaws are consolidated for convenience only and are merely representative. Each consolidated bylaw consists of the original bylaw text and maps, together with current amendments which have been made to the original version. Copies of all bylaws (original and amendments) may be obtained from the SLRD Planning and Development Department. BY-LAW NO. DATE OF ADOPTION 1008 - 2008 Major Review of Area C OCP April 27, 2009 Creating a Medical Marihuana Production Facility 1311 – 2014 October 27, 2014 Development Permit Area Designating the Whistler Waldorf School at WedgeWoods as 1368-2014 March 18, 2015 Institutional Page | 2 Area C Official Community Plan Bylaw No. 689 Official Community Plan Bylaw 689 (Consolidated for Convenience Only) March 2015 SQUAMISH LILLOOET REGIONAL DISTRICT BYLAW NO. 1008, 2008 A by-law of the Squamish Lillooet Regional District to amend the Electoral Area C Official Community Plan Bylaw No. 689, 1999. WHEREAS the Board of the Squamish Lillooet Regional District wishes to adopt a new Electoral Area C Official Community Plan; NOW THEREFORE, the Regional Board of the Squamish Lillooet Regional District, in open meeting assembled, enacts as follows: 1. -

Community Risk Assessment
COMMUNITY RISK ASSESSMENT Squamish-Lillooet Regional District Abstract This Community Risk Assessment is a component of the SLRD Comprehensive Emergency Management Plan. A Community Risk Assessment is the foundation for any local authority emergency management program. It informs risk reduction strategies, emergency response and recovery plans, and other elements of the SLRD emergency program. Evaluating risks is a requirement mandated by the Local Authority Emergency Management Regulation. Section 2(1) of this regulation requires local authorities to prepare emergency plans that reflects their assessment of the relative risk of occurrence, and the potential impact, of emergencies or disasters on people and property. SLRD Emergency Program [email protected] Version: 1.0 Published: January, 2021 SLRD Community Risk Assessment SLRD Emergency Management Program Executive Summary This Community Risk Assessment (CRA) is a component of the Squamish-Lillooet Regional District (SLRD) Comprehensive Emergency Management Plan and presents a survey and analysis of known hazards, risks and related community vulnerabilities in the SLRD. The purpose of a CRA is to: • Consider all known hazards that may trigger a risk event and impact communities of the SLRD; • Identify what would trigger a risk event to occur; and • Determine what the potential impact would be if the risk event did occur. The results of the CRA inform risk reduction strategies, emergency response and recovery plans, and other elements of the SLRD emergency program. Evaluating risks is a requirement mandated by the Local Authority Emergency Management Regulation. Section 2(1) of this regulation requires local authorities to prepare emergency plans that reflect their assessment of the relative risk of occurrence, and the potential impact, of emergencies or disasters on people and property. -

Current and Future Snow Avalanche Threats and Mitigation Measures in Canada
CURRENT AND FUTURE SNOW AVALANCHE THREATS AND MITIGATION MEASURES IN CANADA Prepared for: Public Safety Canada Prepared by: Cam Campbell, M.Sc.1 Laura Bakermans, M.Sc., P.Eng.2 Bruce Jamieson, Ph.D., P.Eng.3 Chris Stethem4 Date: 2 September 2007 1 Canadian Avalanche Centre, Box 2759, Revelstoke, B.C., Canada, V0E 2S0. Phone: (250) 837-2748. Fax: (250) 837-4624. E-mail: [email protected] 2 Department of Civil Engineering, University of Calgary, 2500 University Drive NW. Calgary, AB, Canada, T2N 1N4, Canada. E-mail: [email protected] 3 Department of Civil Engineering, University of Calgary, 2500 University Drive NW. Calgary, AB, Canada, T2N 1N4, Canada. Phone: (403) 220-7479. Fax: (403) 282-7026. E-mail: [email protected] 4 Chris Stethem and Associates Ltd., 120 McNeill, Canmore, AB, Canada, T1W 2R8. Phone: (403) 678-2477. Fax: (403) 678-346. E-mail: [email protected] Table of Contents EXECUTIVE SUMMARY This report presents the results of the Public Safety Canada funded project to inventory current and predict future trends in avalanche threats and mitigation programs in Canada. The project also updated the Natural Resources Canada website and map of fatal avalanche incidents. Avalanches have been responsible for at least 702 fatalities in Canada since the earliest recorded incident in 1782. Sixty-one percent of these fatalities occurred in British Columbia, with 13% in Alberta, 11% in Quebec and 10% in Newfoundland and Labrador. The remainder occurred in Ontario, Nova Scotia and the Yukon, Northwest and Nunavut Territories. Fifty-three percent of the fatalities were people engaged in recreational activities, while 18% were people in or near buildings, 16% were travelling or working on transportation corridors and 8% were working in resource industries. -

Gates Creek Salmonid Population Assessment, Spring and Summer 2016
Gates Creek Salmonid Population Assessment, Spring and Summer 2016 Implementation Year 5 (2016): FWCP Project No: COA-F17-1360 Reference: GATESCHAN 0009 Stephanie Lingard, Daniel Ramos- Espinoza, Nicholas Burnett, Douglas Braun and Caroline Melville Prepared for: The Fish and Wildlife Compensation Program Lillooet Tribal Council Fisheries and Oceans Canada Prepared by: InStream Fisheries Research Inc. 1211A Enterprise Way Squamish BC, V8B 0E8 Report prepared with financial support of the Fish and Wildlife Compensation Program on behalf of its program partners BC Hydro, the Province of BC, First Nation and Public Stake Holders and Fisheries and Oceans Canada. Executive Summary This report summarizes the monitoring activities of the Gates Creek Salmonid Habitat and Population Assessment. The project was funded by the Fish and Wildlife Compensation Program. The Gates Creek Salmonid Habitat and Population Assessment primarily addresses the "research and information acquisition" action in the Bridge Seton Salmonid Action Plan by providing baseline population estimates for rearing salmonids in the Gates Creek watershed. This report is broken into two chapters to accommodate the difference in methodologies used between the two field components of the project. The “Gates Creek Juvenile Sockeye Salmon Migration and Survival” is the first chapter and discusses the final year of data collection for juvenile Sockeye Salmon that commenced in the spring of 2012. The chapter summarizes the abundance and survival estimates for Sockeye Salmon fry leaving Gates Creek and the Gates Creek spawning channel over the past five years with emphasis on the data collected in the spring of 2016. The second chapter, the “Gates Creek Juvenile Salmonid Assessment” presents the results of the first year of a proposed four-year open site electrofishing mark-recapture survey for juvenile Coho Salmon, Bull Trout and Rainbow Trout. -

Geographical Codes Canada - British Columbia (BC)
BELLCORE PRACTICE BR 751-401-161 ISSUE 17, FEBRUARY 1999 COMMON LANGUAGE® Geographical Codes Canada - British Columbia (BC) BELLCORE PROPRIETARY - INTERNAL USE ONLY This document contains proprietary information that shall be distributed, routed or made available only within Bellcore, except with written permission of Bellcore. LICENSED MATERIAL - PROPERTY OF BELLCORE Possession and/or use of this material is subject to the provisions of a written license agreement with Bellcore. Geographical Codes Canada - British Columbia (BC) BR 751-401-161 Copyright Page Issue 17, February 1999 Prepared for Bellcore by: R. Keller For further information, please contact: R. Keller (732) 699-5330 To obtain copies of this document, Regional Company/BCC personnel should contact their company’s document coordinator; Bellcore personnel should call (732) 699-5802. Copyright 1999 Bellcore. All rights reserved. Project funding year: 1999. BELLCORE PROPRIETARY - INTERNAL USE ONLY See proprietary restrictions on title page. ii LICENSED MATERIAL - PROPERTY OF BELLCORE BR 751-401-161 Geographical Codes Canada - British Columbia (BC) Issue 17, February 1999 Trademark Acknowledgements Trademark Acknowledgements COMMON LANGUAGE is a registered trademark and CLLI is a trademark of Bellcore. BELLCORE PROPRIETARY - INTERNAL USE ONLY See proprietary restrictions on title page. LICENSED MATERIAL - PROPERTY OF BELLCORE iii Geographical Codes Canada - British Columbia (BC) BR 751-401-161 Trademark Acknowledgements Issue 17, February 1999 BELLCORE PROPRIETARY - INTERNAL USE ONLY See proprietary restrictions on title page. iv LICENSED MATERIAL - PROPERTY OF BELLCORE BR 751-401-161 Geographical Codes Canada - British Columbia (BC) Issue 17, February 1999 Table of Contents COMMON LANGUAGE Geographic Codes Canada - British Columbia (BC) Table of Contents 1. -

One Mile Lake Environmental Conditions Report
One Mile Lake Environmental Conditions Report Prepared for: Village Of Pemberton PO Box 100 Pemberton, BC V0N 2L0 October 2, 2015 Prepared by: Veronica Woodruff, Dipl. Tech. Stewardship Pemberton Society One Mile Lake Environmental Condition Report Page ii TABLE OF CONTENTS TABLE OF CONTENTS ....................................................................................................... II LIST OF FIGURES ................................................................................................................ III 1. INTRODUCTION ............................................................................................................. 4 2. HISTORY ........................................................................................................................... 4 3. CURRENT ENVIRONMENTAL CONDITIONS .......................................................... 7 3.1. WATERSHED ........................................................................................................................................ 7 3.2. BIOGEOCLIMATIC ZONE AND ECOSYSTEMS ................................................................................... 7 3.3. SPECIES PRESENT ................................................................................................................................ 8 3.3.1. Fish ................................................................................................................................................. 8 3.3.2. Birds .............................................................................................................................................. -
Regional Destination Development Strategy
VANCOUVER, COAST & MOUNTAINS REGIONAL DESTINATION DEVELOPMENT STRATEGY 2019–2029 HOPE DESTINATION BC REPRESENTATIVES Photo: Hubert Kang Jody Young MANAGER, VANCOUVER, COAST & MOUNTAINS AND INDUSTRY PROGRAMS Jonathan Heerema SENIOR PROJECT ADVISOR, DESTINATION DEVELOPMENT [email protected] INDIGENOUS TOURISM ASSOCIATION OF BC 604 921 1070 [email protected] MINISTRY OF TOURISM, ARTS AND CULTURE Amber Mattock SENIOR DIRECTOR 250 356 1489 [email protected] TABLE OF CONTENTS I. EXECUTIVE SUMMARY ........................................................1 4. A DISTINCTIVE DIRECTION ..........................................24 4.1 Vision II. ACRONYMS ...........................................................................5 4.2 Goals 1. FOREWORD AND ACKNOWLEDGEMENTS ................. 7 4.3 Guiding principles 4.4 Development themes 2. INTRODUCING THE STRATEGY .......................................9 A Focus on the Supply and Visitor Experience 5. VANCOUVER, COAST & MOUNTAINS STRATEGIC PRIORITIES ...................................................26 Program Vision and Goals 5.1 Development Theme 1 Purpose 5.2 Development Theme 2 Methodology 5.3 Development Theme 3 Alignment 5.4 Development Theme 4 3. VANCOUVER, COAST 5.5 Development Theme 5 & MOUNTAINS OVERVIEW ............................................. 14 3.1 Introduce the region 6. IMPLEMENTATION FRAMEWORK ................................35 3.2 Map of Vancouver, Coast & Mountains 7. MEASURING AND MONITORING SUCCESS .............36 3.3 Visitor Markets 3.4 Summary -

Squamish Valley
S- Coronary care policy adopted Hospital board trustee adopted development of severe at- a policy which clarifies coronary rhythd. care management in the hospital The admission and manage: at the annual meeting Thursday rnent of coronary cases may be in- night. fluenced by the availability of ' The policy is based on the both nursingandmedicalperson- following considerations: ne1 and also the availability of *transfer time, one hour equipment in the hospital. minimum between Squamish and The patient will be admitted the nearest major hospital, and treated at the discretion of the represents a considerable risk to physicians. the patient, particularly with The guidelines for the physi- respect to arrhythmias (irregular cians and nurse include criteria for heart beat) during transfer. admission, initial management *the ability to treat the patient and evaluation of the patient, con- in his or her own locality is impor- tinued cardiac monitoring and tant for both patient ahd family medication management protocol. convenience. The patient may have his or her own physician and nurses who arc.-. .. *Some studies would indicate similar morbidity fwes compar- ing small local hospitals to large Few accide tertiary care centres. *the majority of patients enter- A of accidents took ing hospital who suspected of place in the past week sending a having a heart attack rqire only few people to hospital with in- 24 hours monitoring before bemg juria. transferred to a general ward for rehabilitation. Op June 13, on Clarke Drive The policy recognize there are David Turnquist of Squamish left certain categories of patients who the road and hit a tree when the will require transfer to tertiary hood of his QU flew up and block- care during their stay for a ed his vision. -

Squamish-Lillooet Regional District Board Meeting Agenda August 27, 2012; 10:30 AM - 2:00 PM SLRD Boardroom 1350 Aster Street, Pemberton BC
Squamish-Lillooet Regional District Board Meeting Agenda August 27, 2012; 10:30 AM - 2:00 PM SLRD Boardroom 1350 Aster Street, Pemberton BC Item Item of Business and Page # Page 1. Call to Order 2. Approval of Agenda 3. Committee Reports and Recommendations 3.1. Electoral Area Directors Committee Recommendations of August 13, 2011: Unweighted All Vote (except as noted) 3.1.1. Request for Decision - Amendment Bylaw 1256-2012 - Changes to 6-9 Clarify Definition of Agricultural Buildings 1. THAT Bylaw No. 1256, cited as ‘Squamish-Lillooet Regional District Electoral Area C Bylaw No. 765, 2002, Amendment Bylaw No. 1256-2012’ be introduced and read a first and second time; 2. THAT as per s. 890(4) of the Local Government Act the public hearing be waived; 3. THAT a notice of waiver of the public hearing for Bylaw No. 1256, cited as ‘Squamish-Lillooet Regional District Electoral Area C Bylaw No. 765, 2002, Amendment Bylaw No. 1256- 2012’ be advertised as per sections 890 and 893 of the Local Government Act. 3.1.2. Request for Decision - Development Variance Permit #103 10-16 Amendment - Pavilion Lake, Electoral Area B - Petroski THAT the Board amend Development Variance Permit #103 by deleting Section 3, and replacing it with the following: Notwithstanding the provisions of Section 6A.2.4 “Minimum Setback” in the RR2A Rural Resource (8ha) Zone of the Area A and B Zoning Bylaw 670-2000, the minimum setback from a side parcel line is decreased by 2.85m from 4.5m to 1.65m as shown on the attached Site Plan identified as Schedule 2 and attached hereto and forming part of this permit. -
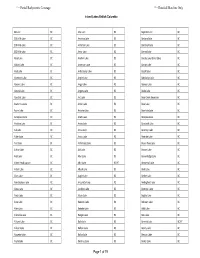
^ = Partial Bathymetric Coverage * = Detailed Shoreline Only Page 1 of 19
^ = Partial Bathymetric Coverage * = Detailed Shoreline Only Inland Lakes British Columbia #3 Lake BC Alta Lake BC Baptiste Lake BC 103 Mile Lake BC Amanita Lake BC Barbara Lake BC 108 Mile Lake BC Ambrose Lake BC Bardolph Lake BC 130 Mile Lake BC Amor Lake BC Barnes Lake BC Abas Lake BC Anahim Lake BC Barsby Lake (Blind Lake) BC Abbott Lake BC Anderson Lake BC Barton Lake BC Abel Lake BC Andy Bailey Lake BC Basalt Lake BC Aberdeen Lake BC Angler Lake BC Battleship Lake BC Abrams Lake BC Angly Lake BC Baynes Lake BC Abruzzi Lake BC Angora Lake BC Beale Lake BC Abuntlet Lake BC Ant Lake BC Bear Creek Reservoir BC Academus Lake BC Antler Lake BC Bear Lake BC Acorn Lake BC Antoine Lake BC Bearhole Lake BC Aeroplane Lake BC Anutz Lake BC Bearpaw Lake BC Ahdatay Lake BC Anzac Lake BC Beartrack Lake BC Aid Lake BC Anzus Lake BC Beartrap Lake BC Aiken Lake BC Arctic Lake BC Beatrice Lake BC Aird Lake BC Armstrong Lake BC Beaux Yeux Lake BC Airline Lake BC Ash Lake BC Beaver Lake BC Alah Lake BC Atan Lake BC Beaverlodge Lake BC Albert Head Lagoon BC Atlin Lake BC/YT Beavertail Lake BC Albert Lake BC Atluck Lake BC Beck Lake BC Alces Lake BC Augier Lake BC Becker Lake BC Alex Graham Lake BC Azouzetta Lake BC Bedingfield Lake BC Alexis Lake BC Azuklotz Lake BC Bednesti Lake BC Aleza Lake BC Azure Lake BC Begbie Lake BC Alice Lake BC Babcock Lake BC Belcourt Lake BC Allan Lake BC Babette Lake BC Bells Lake BC Allendale Lake BC Badger Lake BC Ben Lake BC Alleyne Lake BC Baile Lake BC Bennett Lake BC/YT Allison Lake BC Balfour Lake BC Benny Lake BC -

Squamish-Lillooet Regional District Regional Growth Strategy Bylaw No
SLRD Regular Meeting Agenda April 26, 2010; 10:30 AM SLRD Boardroom 1350 Aster Street, Pemberton BC Item Item of Business and Recommended Action Page Action Info 1 Call to Order 2 Approval of Agenda 3 Committee Reports and Recommendations 3.1 Electoral Area Directors Committee Recommendations of April n/a 12, 2010: 1. Non-Farm Use in the ALR Squamish Valley, Area D Bass Coast Music Festival 1. THAT the request for reconsideration of the application for a non-farm use in the ALR, submitted by Bass Coast Projects Ltd. in 2009 for the lands legally described as DL 994, NWD to allow the festival for 4 days per year, on an annual basis, be forwarded to the Agricultural Land Commission (ALC) with a recommendation of support on the condition that the festival remain in substantial compliance with the proposal submitted in 2009. 2. THAT a new application be submitted for consideration by the SLRD and ALC if the festival is no longer in substantial compliance with the proposal as submitted in 2009. See item 5.1.12 for Staff recommended amended resolution 2. Sea-to-Sky Air Quality Management Plan Financial Contribution for 2010 THAT the SLRD Board continue to fund the Sea-to-Sky Air Quality Management Plan and authorize a cash contribution of $659 for 2010. 3. Village of Pemberton Referral of OCP and Zoning Amendment Applications THAT the Village of Pemberton be advised that with respect to their proposed zoning and OCP amendment, the interests of the SLRD are not affected, and no further consultation is necessary, however, the application itself has technical details that need to be SLRD Regular Meeting Agenda - 2 - April 26, 2010 Item Item of Business and Recommended Action Page Action Info addressed by the Village of Pemberton. -

CHAPTER 20 Holocene Human Occupation and Use of the Slapus
CHAPTER 20 Holocene Human Occupation and Use of the Slapus/Lelachen Locality in the Lower Lillooet River Valley Adrian J. Sanders Rhizome Cultural Heritage Research and Consulting Peter Merchant University of British Columbia Introduction and Background periphery of the Coast and Interior Plateau Salish worlds, a The Lower Lillooet River valley was far from a cultural “transitional zone between the Northwest Coast and Interior side-show to the Lower and Central Fraser River, and Salish Plateau Culture Areas” (Ferguson and Brolly 2011:7); a Sea nexus. Rather, it appears that in terms of longevity and place from which people migrated to the core of cultural intensity of occupation, it rivaled its more celebrated homelands following the catastrophic epidemics of the 18th neighbors to the south, and north/northeast. This chapter and 19th centuries (Carlson 2010:98-99). addresses this fact by drawing on oral traditions, historic and ethnographic accounts, and results of four archaeological projects conducted at the supra-village locality of Slapus, Lelachen and Quqéwelq (Figure 1) between 1999 and 2013 (Hudson and DePaoli 1999; Hudson 2000, 2001, 2003; Wells et al. 2009; Ferguson and Brolly 2011; Sanders and Merchant 2016). Accumulated data suggests that the cultural landscape comprising this locale was continuously and intensively occupied from the early Holocene through the colonial era, and into the present, situating this primary supra-village among the most significant archaeological complexes within the Salish world. We maintain that the Lower Lillooet was a key foci of Salishan cultural florescence, and there is much to benefit through reconsidering the ways in which this nexus was complimentary to those in the Middle and Lower Fraser River and Salish Sea, which to date, espouse the accepted cultural development narrative.