Essentials of Pain Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydromorphone
Hydromorphone WHAT IS HYDROMORPHONE? sedation, and reduced anxiety. It may also cause Hydromorphone belongs to a class of drugs mental clouding, changes in mood, nervousness, called “opioids,” which includes morphine. It and restlessness. It works centrally (in the has an analgesic potency of two to eight times brain) to reduce pain and suppress cough. greater than that of morphine and has a rapid Hydromorphone use is associated with both onset of action. physiological and psychological dependence. WHAT IS ITS ORIGIN? What is its effect on the body? Hydromorphone is legally manufactured and Hydromorphone may cause: distributed in the United States. However, • Constipation, pupillary constriction, urinary retention, users can obtain hydromorphone from nausea, vomiting, respiratory depression, dizziness, forged prescriptions, “doctor-shopping,” impaired coordination, loss of appetite, rash, slow or theft from pharmacies, and from friends and rapid heartbeat, and changes in blood pressure acquaintances. What are its overdose effects? What are the street names? Acute overdose of hydromorphone can produce: Common street names include: Severe respiratory depression, drowsiness • D, Dillies, Dust, Footballs, Juice, and Smack progressing to stupor or coma, lack of skeletal muscle tone, cold and clammy skin, constricted What does it look like? pupils, and reduction in blood pressure and heart Hydromorphone comes in: rate • Tablets, capsules, oral solutions, and injectable Severe overdose may result in death due to formulations respiratory depression. How is it abused? Which drugs cause similar effects? Users may abuse hydromorphone tablets by Drugs that have similar effects include: ingesting them. Injectable solutions, as well as • Heroin, morphine, hydrocodone, fentanyl, and tablets that have been crushed and dissolved oxycodone in a solution may be injected as a substitute for heroin. -

1 Impact of Opioid Agonists on Mental Health in Substitution
Impact of opioid agonists on mental health in substitution treatment for opioid use disorder: A systematic review and Bayesian network meta-analysis of randomized clinical trials Supplementary Table 1_ Specific search strategy for each database The following general combination of search terms, Boolean operators, and search fields were used where “*” means that any extension of that word would be considered: Title field [opium OR opiate* OR opioid OR heroin OR medication assisted OR substitution treatment OR maintenance treatment OR methadone OR levomethadone OR buprenorphine OR suboxone OR (morphine AND slow) OR diamorphine OR diacetylmorphine OR dihydrocodeine OR hydromorphone OR opium tincture OR tincture of opium OR methadol OR methadyl OR levomethadyl] AND Title/Abstract field [trial* OR random* OR placebo] AND All fields [depress* OR anxiety OR mental] Wherever this exact combination was not possible, a more inclusive version of the search strategy was considered. Database Search Strategy Ovid for EBM Reviews - Cochrane Central Register of (opium or opiate$ or opioid or heroin or medication Controlled Trials August 2018; Embase 1974 to assisted or substitution treatment or maintenance September 07, 2018; MEDLINE(R) and Epub Ahead treatment or methadone or levomethadone or of Print, In-Process & Other Non-Indexed Citations buprenorphine or suboxone or (morphine and slow) or and Daily 1946 to September 07, 2018 diamorphine or diacetylmorphine or dihydrocodeine or hydromorphone or opium tincture or tincture of opium or methadol or methadyl -

Hydromorphone and Morphine Are Not the Same Drug!
HYDROmorphone and Morphine are not the same drug! HYDROmorphone and morphine are deemed high risk/high alert medications. An Independent Double Check (IDC) must be done to avoid errors when administering all high risk drugs. Please refer to Clinical Practice Standard 1-20-6-3-260. HYDROmorphone and morphine need to knows: HYDROmorphone is 5 times more potent than morphine! (e.g., 1 mg of HYDROmorphone PO is equivalent to 5 mg of morphine PO) When administered via the subcutaneous route, these drugs are twice as strong! Therefore when converting between oral and subcutaneous routes half the dose. (10 mg of oral morphine = 5 mg of subcutaneous morphine) HYDROmorphone and morphine: The name game. Both drugs are used in the management of moderate to severe pain. HYDROmorphone and morphine come in different brand names and formulations. HYDROmorphone = Dilaudid (Injectable), Jurnista (SR), HYDROmorph Contin (SR) Morphine = Morphine Sulphate (Injectable), MS-IR, M.O.S. (Liquid/Tablet/SR), M-Eslon (SR), Statex (Liquid/Tablet/Supp.), Doloral (Liquid), MS Contin (SR), Kadian (SR) (SR=Slow Release CAP) *As per NH, use the generic names (NOT THE BRAND NAME), of these drugs in your documentation. Common side effects: 3 ‘B’s of opioid prescribing: Common: Nausea, Vomiting, Constipation, Sedation, Syncope, Xerostomia (Dry Mouth), If your client is on an opioid: Delirium, Restlessness, Urinary Retention. Do they have a Breakthrough? Less Common: Respiratory Depression, Do they have something for Barfing? Opioid-Induced Neurotoxicity, Myoclonus, Do they have something for their Bowels? Pruritis (itching). Opioid induced neurotoxicity: What does it look like? Opioid induced neurotoxicity is the result of a build up of toxic opioid metabolites in the blood stream. -

Do You Know... Methadone
Do You Know... Street names: juice, meth (also used to refer to methamphetamines) What is it? Methadone belongs to the opioid family of Methadone drugs. It is used most commonly to treat addiction to other opioid drugs such as heroin, oxycodone (e.g., Percodan, Percocet), fentanyl (e.g., Duragesic, Sublimaze) and hydromorphone (e.g., Dilaudid). Methadone is a synthetic opioid, which means that it is made from chemicals in a lab. Methadone was developed in Germany during the Second World War and was first used to provide pain relief. Methadone maintenance treatment, which prevents opioid withdrawal and reduces or eliminates drug cravings, was first developed in the 1960s. For many years, Canadian reg- ulations around the prescription of methadone were so restrictive that few doctors offered the treatment. People who wanted methadone treatment often had to wait months or years. 1/4 © 2003, 2011, 2012 CAMH | www.camh.ca In the 1990s, the need to reduce the harm of drug use with medical care, improves the chances of having a was more clearly recognized, and changes were made healthy baby. There are no known long-term effects of to make it easier for doctors to provide methadone methadone on the baby. treatment. People who inject opioid drugs regularly, and who are Methadone maintenance is not a “cure”: it is a treatment. HIV- or hepatitis C–positive, are enrolled in methadone Through treatment, people who are addicted to opioids treatment to help protect their health. Methadone receive the medical and social support they need to treatment also helps to prevent these infections from stabilize and improve their lives. -

Hydromorphone Hydrochloride) CS-II WARNING: DILAUDID ORAL LIQUID and DILAUDID TABLETS CONTAIN HYDROMORPHONE, WHICH IS a POTENT SCHEDULE II CONTROLLED OPIOID AGONIST
DILAUDID® ORAL LIQUID and DILAUDID® TABLETS (hydromorphone hydrochloride) CS-II WARNING: DILAUDID ORAL LIQUID AND DILAUDID TABLETS CONTAIN HYDROMORPHONE, WHICH IS A POTENT SCHEDULE II CONTROLLED OPIOID AGONIST. SCHEDULE II OPIOID AGONISTS, INCLUDING MORPHINE, OXYMORPHONE, OXYCODONE, FENTANYL, AND METHADONE, HAVE THE HIGHEST POTENTIAL FOR ABUSE AND RISK OF PRODUCING RESPIRATORY DEPRESSION. ALCOHOL, OTHER OPIOIDS AND CENTRAL NERVOUS SYSTEM DEPRESSANTS (SEDATIVE-HYPNOTICS) POTENTIATE THE RESPIRATORY DEPRESSANT EFFECTS OF HYDROMORPHONE, INCREASING THE RISK OF RESPIRATORY DEPRESSION THAT MIGHT RESULT IN DEATH. DESCRIPTION Proprietary name: DILAUDID ORAL LIQUID Established name: hydromorphone hydrochloride Route of administration: ORAL (C38288) Active ingredients (moiety): hydromorphone hydrochloride (hydromorphone) # Strength Form Inactive ingredients 1 5 MILLIGRAM LIQUID purified water, methylparaben, propylparaben, sucrose, glycerin, sodium (C42953) metabisulfite Proprietary name: DILAUDID TABLETS Established name: hydromorphone hydrochloride Route of administration: ORAL (C38288) Active ingredients (moiety): hydromorphone hydrochloride (hydromorphone) # Strength Form Inactive ingredients 1 2 MILLIGRAM TABLET D&C red #30 Lake dye, D&C yellow #10 Lake dye, lactose, magnesium (C42998) stearate, sodium metabisulfite 2 4 MILLIGRAM TABLET D&C yellow #10 Lake dye, lactose, magnesium stearate, sodium metabisulfite (C42998) 3 8 MILLIGRAM TABLET lactose anhydrous, magnesium stearate, sodium metabisulfite (C42998) DILAUDID (hydromorphone hydrochloride), a hydrogenated ketone of morphine, is an opioid analgesic. The chemical name of DILAUDID (hydromorphone hydrochloride) is 4,5α-epoxy-3- hydroxy-17-methylmorphinan-6-one hydrochloride. The structural formula is: M.W. 321.8 Each 5 mL (1 teaspoon) of DILAUDID ORAL LIQUID contains 5 mg of hydromorphone hydrochloride. In addition, other ingredients include purified water, methylparaben, propylparaben, sucrose, and glycerin. DILAUDID ORAL LIQUID may contain traces of sodium metabisulfite. -
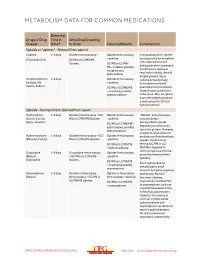
Metabolism Data for Common Medications
METABOLISM DATA FOR COMMON MEDICATIONS Detection Drugs or Drug Time in Urine Drug Screening Classes Urine* to Order Expected Results Consideration Opioids or “opiates” – Natural (from opium) Codeine 1–3 days Opiates Immunoassay + Opiates Immunoassay Immunoassays for “opiates” are responsive for morphine (Tylenol #2/3/4) GC/MS or LC/MS/MS – positive and codeine but do not Opiates GC/MS or LC/MS/ distinguish which is present. MS – codeine, possibly Confirmatory testing is morphine and required to reliably identify hydrocodone drug(s) present. Since Morphine (Avinza, 1–3 days Opiates Immunoassay codeine is metabolized Embeda, MS – positive to morphine and small Contin, Kadian) GC/MS or LC/MS/MS quantities to hydrocodone, – morphine, possibly these drugs may be found hydromorphone in the urine. Also, morphine may metabolize to produce a small amount (<10%) of hydromorphone. Opioids – Semisynthetic (derived from opium Hydrocodone 1–3 days Opiates Immunoassay + GC/ Opiates Immunoassay “Opiates” immunoassays (Lorcet, Lortab, MS or LC/MS/MS Opiates – positive may also detect Norco, Vicodin) semisynthetic opioids GC/MS or LC/MS/MS – depending on their cross- hydrocodone, possibly reactivity pattern. However, hydromorphone a negative result does not Hydromorphone 1–3 days Opiates Immunoassay + GC/ Opiates Immunoassay exclude use of semisynthetic (Dilaudid, Exalgo) MS or LC/MS/MS Opiates –positive opioids. Confirmatory GC/MS or LC/MS/MS testing (GC/MS or LC/ – hydromorphone MS/MS) is required to verify compliance with the Oxycodone 1–3 days Oxycodone Immunoassay Opiates Immunoassay prescribed semisynthetic (Roxicet, + GC/MS or LC/MS/MS –positive opioid(s). OxyContin) Opiates GC/MS or LC/MS/MS Since hydrocodone is – oxycodone possibly metabolized in small oxymorphone amounts to hydromorphone, Oxymorphone 1–3 days Opiates or Oxycodone Opiates or Oxycodone both may be found in (Opana) Immunoassay + GC/MS or Immunoassay – positive the urine. -

Hydromorphone Hydrochloride) C-II
NDA 19-034/S-018-Label Page 1 DILAUDID® and DILAUDID-HP® INJECTION 1 mg/mL, 2 mg/mL, 4mg/mL, and 10 mg/mL (hydromorphone hydrochloride) C-II WARNING: DILAUDID-HP (high potency, 10 mg/mL ampules and vials) is a more concentrated solution of hydromorphone than DILAUDID INJECTION, and is intended for use only in opioid-tolerant patients. Do not confuse DILAUDID-HP with standard parenteral formulations of DILAUDID or other opioids, as overdose and death could result. DILAUDID® INJECTION (1, 2, and 4 mg/mL ampules, sterile solution for parenteral administration) and DILAUDID-HP® contain hydromorphone, a potent Schedule II opioid agonist. Schedule II opioid agonists, including morphine, oxymorphone, hydromorphone, oxycodone, fentanyl and methadone, have the highest potential for abuse and risk of producing respiratory depression. Ethanol, other opioids, and other central nervous system depressants (e.g., sedative-hypnotics, skeletal muscle relaxants) can potentiate the respiratory- depressant effects of hydromorphone and increase the risk of adverse outcome, including death. DESCRIPTION DILAUDID (hydromorphone hydrochloride), a hydrogenated ketone of morphine, is an opioid analgesic. The chemical name of DILAUDID (hydromorphone hydrochloride) is 4,5α-epoxy-3-hydroxy-17-methylmorphinan-6-one hydrochloride. The structural formula is: NDA 19-034/S-018-Label Page 2 M.W. 321.8 DILAUDID INJECTION is available in ampules for parenteral administration. Each 1 mL of sterile solution contains 1 mg, 2 mg, or 4 mg hydromorphone hydrochloride with 0.2% sodium citrate and 0.2% citric acid solution. DILAUDID INJECTION ampules are sterile. HIGH POTENCY DILAUDID (DILAUDID-HP) is available in AMBER ampules or single dose vials for intravenous (IV), subcutaneous (SC), or intramuscular (IM) administration. -

Use of Chronic Opioid Therapy in Chronic Noncancer Pain CHRONIC NONCANCER PAINNONCANCER CHRONIC Evidence Review
CLINICAL GUIDELINE FOR THE USE OF CHRONIC OPIOID THERAPY IN CLINICAL GUIDELINE FOR THE USE OF CHRONIC OPIOID THERAPY IN GUIDELINE FOR THE Use of Chronic Opioid Therapy in Chronic Noncancer Pain CHRONIC NONCANCER PAIN Evidence Review The American Pain Society in Conjunction with The American Academy of Pain Medicine EVIDENCE REVIEW APS-AAPM Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain TABLE OF CONTENTS Page Introduction 1 Purpose of evidence review ...................................................................... 1 Background 1 Previous guidelines ................................................................................... 2 Scope of evidence review 3 Key questions............................................................................................ 3 Populations................................................................................................ 7 Interventions.............................................................................................. 8 Outcomes.................................................................................................. 8 Conflict of interest............................................................................................. 10 Methods 10 Literature search and strategy................................................................... 10 Inclusion and exclusion criteria.................................................................. 11 Data extraction and synthesis .................................................................. -

Medications: Opioid
Opioid Medications: Dosages, Adverse Events, and Patient Education APhA Pain Institute Opioid Medications: Dosages, Adverse Events, and Patient Education Opioid Mechanisms Learning Objectives Mu, delta, and kappa are the three receptors involved in the effects At the completion of this knowledge-based activity, of opioids, including both their analgesic activity and their adverse participants will be able to: effects. The mu-opioid receptor is most commonly responsible for analgesia, respiratory depression, and euphoria. The kappa-opioid 1. Identify the relative efficacy of various opioids for pain receptor can be responsible for dysphoria or analgesia depending management. on which receptor subtype is activated. Opioids also exhibit varying 2. Describe common adverse events associated with available activity at these receptor sites as agonists, antagonists, and partial opioids for pain management. agonists or mixed agonist/antagonist properties. 3. Discuss patient counseling and education strategies that support As the dose of a pure opioid agonist increases, analgesic effects appropriate use of opioids. increase but so does the risk of respiratory depression due to carbon dioxide accumulation. Partial agonists/antagonists, such as Background buprenorphine, are unique in that as the dose increases, carbon dioxide accumulation and the associated risk of respiratory The release of the Centers for Disease Control and Prevention depression plateaus.2 This could represent a potentially safer option (CDC) guidelines for prescribing opioids has brought national for patients with comorbid respiratory conditions. Buprenorphine attention to opioid prescribing in the primary care setting. These is also associated with decreased euphoria due to these mixed guidelines include specific qualifying parameters for initiating partial agonist/antagonist properties, which could offer a safe and and continuing opioids intended for long-term use in patients with effective treatment in patients with a history of “dose creeping” and 1 chronic noncancer pain. -

Replacement of Current Opioid Drugs Focusing on MOR-Related Strategies
JPT-107519; No of Pages 17 Pharmacology & Therapeutics xxx (2020) xxx Contents lists available at ScienceDirect Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/pharmthera Replacement of current opioid drugs focusing on MOR-related strategies Jérôme Busserolles a,b, Stéphane Lolignier a,b, Nicolas Kerckhove a,b,c, Célian Bertin a,b,c, Nicolas Authier a,b,c, Alain Eschalier a,b,⁎ a Université Clermont Auvergne, INSERM, CHU, NEURO-DOL Pharmacologie Fondamentale et Clinique de la douleur, F-63000 Clermont-Ferrand, France b Institut ANALGESIA, Faculté de Médecine, F-63000 Clermont-Ferrand, France c Observatoire Français des Médicaments Antalgiques (OFMA), French monitoring centre for analgesic drugs, CHU, F-63000 Clermont-Ferrand, France article info abstract Available online xxxx The scarcity and limited risk/benefit ratio of painkillers available on the market, in addition to the opioid crisis, warrant reflection on new innovation strategies. The pharmacopoeia of analgesics is based on products that are often old and derived from clinical empiricism, with limited efficacy or spectrum of action, or resulting in Keywords: an unsatisfactory tolerability profile. Although they are reference analgesics for nociceptive pain, opioids are sub- Analgesia ject to the same criticism. The use of opium as an analgesic is historical. Morphine was synthesized at the begin- Mu opioid receptors (MORs) ning of the 19th century. The efficacy of opioids is limited in certain painful contexts and these drugs can induce Opioid adverse side effects potentially serious and fatal adverse effects. The current North American opioid crisis, with an ever-rising number Opioid abuse and misuse of deaths by opioid overdose, is a tragic illustration of this. -

Federal Register/Vol. 85, No. 230/Monday, November 30, 2020
76604 Federal Register / Vol. 85, No. 230 / Monday, November 30, 2020 / Notices notifications disclosing all changes in Act on September 18, 2020 (85 FR DEPARTMENT OF JUSTICE membership. 58390). Drug Enforcement Administration On September 10, 2018, CONFERS Suzanne Morris, [Docket No. DEA–688E] filed its original notification pursuant to Chief, Premerger and Division Statistics, Section 6(a) of the Act. The Department Antitrust Division. Established Aggregate Production of Justice published a notice in the [FR Doc. 2020–26362 Filed 11–27–20; 8:45 am] Federal Register pursuant to Section Quotas for Schedule I and II Controlled BILLING CODE P Substances and Assessment of 6(b) of the Act on October 19, 2018 (83 Annual Needs for the List I Chemicals FR 53106). Ephedrine, Pseudoephedrine, and The last notification was filed with DEPARTMENT OF JUSTICE Phenylpropanolamine for 2021 the Department on May 1, 2020. A notice was published in the Federal Antitrust Division AGENCY: Drug Enforcement Register pursuant to Section 6(b) of the Administration, Department of Justice. Act on May 28, 2020 (85 FR 32049). Notice Pursuant to the National ACTION: Final order. Cooperative Research and Production Suzanne Morris, Act of 1993—Cooperative Research SUMMARY: This final order establishes Chief, Premerger and Division Statistics, Group on AC2AT–II the initial 2021 aggregate production Antitrust Division. quotas for controlled substances in [FR Doc. 2020–26358 Filed 11–27–20; 8:45 am] Notice is hereby given that, on schedules I and II of the Controlled Substances Act and the assessment of BILLING CODE 4410–11–P November 19, 2020, pursuant to Section 6(a) of the National Cooperative annual needs for the list I chemicals Research and Production Act of 1993, ephedrine, pseudoephedrine, and DEPARTMENT OF JUSTICE 15 U.S.C. -

WADA Technical Letter – TL15 HYDROMORPHONE
WADA Technical Letter – TL15 TL15 Document Number: Version Number: 2.0 (replaces TL2018/04) Written by: WADA LabEG Approved by: WADA LabEG* Date: 06 March 2018 Effective Date: 06 March 2018 *The approval by the WADA Executive Committee is applicable only to Technical Letters issued after November 2019. HYDROMORPHONE The World Anti-Doping Agency wishes to draw the attention of the Laboratories to the following issue that may affect Laboratory operations. This pertains, in particular, to the possible detection of the prohibited narcotic Hydromorphone in urine Samples resulting from the administration of the permitted drug Hydrocodone or resulting from the administration of high doses of either the Prohibited Threshold Substance Morphine or the permitted drug Codeine. 1. Detection of Hydromorphone as a result of the administration of Hydrocodone It is indeed reported in the literature1 that Hydrocodone is metabolized by O-demethylation to Hydromorphone and by N-demethylation to Norhydrocodone (Figure 1). In single-dose administration studies 1,2 of Hydrocodone, it was found that the levels of Norhydrocodone in urine were always higher than or equal to the parent compound, whereas the levels of Hydromorphone were lower. Additionally, Norhydrocodone was detected in urine for a longer time than Hydromorphone. In a different study involving a population of 25,200 subjects treated for chronic pain by multiple doses of hydrocodone/acetaminophen formulation3, Barakat et al. showed that the metabolic Hydromorphone/Hydrocodone ratio varied between 7.4 and 35.1%. Figure 1: Metabolic Pathway of Hydrocodone (Adapted from Valtier and Bebarta1) 1 Valtier S and Bebarta VS. Excretion Profile of Hydrocodone, Hydromorphone and Norhydrocodone in Urine Following Single Dose Administration of Hydrocodone to Healthy Volunteers.