Dual Origin and Segmental Organisation of Avian Scapula 3791 Corresponding Embryos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bone Limb Upper
Shoulder Pectoral girdle (shoulder girdle) Scapula Acromioclavicular joint proximal end of Humerus Clavicle Sternoclavicular joint Bone: Upper limb - 1 Scapula Coracoid proc. 3 angles Superior Inferior Lateral 3 borders Lateral angle Medial Lateral Superior 2 surfaces 3 processes Posterior view: Acromion Right Scapula Spine Coracoid Bone: Upper limb - 2 Scapula 2 surfaces: Costal (Anterior), Posterior Posterior view: Costal (Anterior) view: Right Scapula Right Scapula Bone: Upper limb - 3 Scapula Glenoid cavity: Glenohumeral joint Lateral view: Infraglenoid tubercle Right Scapula Supraglenoid tubercle posterior anterior Bone: Upper limb - 4 Scapula Supraglenoid tubercle: long head of biceps Anterior view: brachii Right Scapula Bone: Upper limb - 5 Scapula Infraglenoid tubercle: long head of triceps brachii Anterior view: Right Scapula (with biceps brachii removed) Bone: Upper limb - 6 Posterior surface of Scapula, Right Acromion; Spine; Spinoglenoid notch Suprspinatous fossa, Infraspinatous fossa Bone: Upper limb - 7 Costal (Anterior) surface of Scapula, Right Subscapular fossa: Shallow concave surface for subscapularis Bone: Upper limb - 8 Superior border Coracoid process Suprascapular notch Suprascapular nerve Posterior view: Right Scapula Bone: Upper limb - 9 Acromial Clavicle end Sternal end S-shaped Acromial end: smaller, oval facet Sternal end: larger,quadrangular facet, with manubrium, 1st rib Conoid tubercle Trapezoid line Right Clavicle Bone: Upper limb - 10 Clavicle Conoid tubercle: inferior -
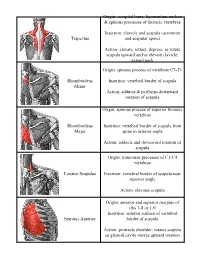
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

A Unified Anatomy Ontology of the Vertebrate Skeletal System
A Unified Anatomy Ontology of the Vertebrate Skeletal System Wasila M. Dahdul1,2*, James P. Balhoff2,3, David C. Blackburn4, Alexander D. Diehl5, Melissa A. Haendel6, Brian K. Hall7, Hilmar Lapp2, John G. Lundberg8, Christopher J. Mungall9, Martin Ringwald10, Erik Segerdell6, Ceri E. Van Slyke11, Matthew K. Vickaryous12, Monte Westerfield11,13, Paula M. Mabee1 1 Department of Biology, University of South Dakota, Vermillion, South Dakota, United States of America, 2 National Evolutionary Synthesis Center, Durham, North Carolina, United States of America, 3 Department of Biology, University of North Carolina, Chapel Hill, North Carolina, United States of America, 4 Department of Vertebrate Zoology and Anthropology, California Academy of Sciences, San Francisco, California, United States of America, 5 The Jacobs Neurological Institute, University at Buffalo, Buffalo, New York, United States of America, 6 Oregon Health and Science University, Portland, Oregon, United States of America, 7 Department of Biology, Dalhousie University, Halifax, Nova Scotia, Canada, 8 Department of Ichthyology, The Academy of Natural Sciences, Philadelphia, Pennsylvania, United States of America, 9 Genomics Division, Lawrence Berkeley National Laboratory, Berkeley, California, United States of America, 10 The Jackson Laboratory, Bar Harbor, Maine, United States of America, 11 Zebrafish Information Network, University of Oregon, Eugene, Oregon, United States of America, 12 Department of Biomedical Sciences, Ontario Veterinary College, University of Guelph, Guelph, -

Comparative Bone Histology of the Turtle Shell (Carapace and Plastron)
Comparative bone histology of the turtle shell (carapace and plastron): implications for turtle systematics, functional morphology and turtle origins Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität zu Bonn Vorgelegt von Dipl. Geol. Torsten Michael Scheyer aus Mannheim-Neckarau Bonn, 2007 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn 1 Referent: PD Dr. P. Martin Sander 2 Referent: Prof. Dr. Thomas Martin Tag der Promotion: 14. August 2007 Diese Dissertation ist 2007 auf dem Hochschulschriftenserver der ULB Bonn http://hss.ulb.uni-bonn.de/diss_online elektronisch publiziert. Rheinische Friedrich-Wilhelms-Universität Bonn, Januar 2007 Institut für Paläontologie Nussallee 8 53115 Bonn Dipl.-Geol. Torsten M. Scheyer Erklärung Hiermit erkläre ich an Eides statt, dass ich für meine Promotion keine anderen als die angegebenen Hilfsmittel benutzt habe, und dass die inhaltlich und wörtlich aus anderen Werken entnommenen Stellen und Zitate als solche gekennzeichnet sind. Torsten Scheyer Zusammenfassung—Die Knochenhistologie von Schildkrötenpanzern liefert wertvolle Ergebnisse zur Osteoderm- und Panzergenese, zur Rekonstruktion von fossilen Weichgeweben, zu phylogenetischen Hypothesen und zu funktionellen Aspekten des Schildkrötenpanzers, wobei Carapax und das Plastron generell ähnliche Ergebnisse zeigen. Neben intrinsischen, physiologischen Faktoren wird die -

Shoulder Shoulder
SHOULDER SHOULDER ⦿ Connects arm to thorax ⦿ 3 joints ◼ Glenohumeral joint ◼ Acromioclavicular joint ◼ Sternoclavicular joint ⦿ https://www.youtube.com/watch?v=rRIz6oO A0Vs ⦿ Functional Areas ◼ scapulothoracic ◼ scapulohumeral SHOULDER MOVEMENTS ⦿ Global Shoulder ⦿ Arm (Shoulder Movement Joint) ◼ Elevation ◼ Flexion ◼ Depression ◼ Extension ◼ Abduction ◼ Abduction ◼ Adduction ◼ Adduction ◼ Medial Rotation ◼ Medial Rotation ◼ Lateral Rotation ◼ Lateral Rotation SHOULDER MOVEMENTS ⦿ Movement of shoulder can affect spine and rib cage ◼ Flexion of arm Extension of spine ◼ Extension of arm Flexion of spine ◼ Adduction of arm Ipsilateral sidebending of spine ◼ Abduction of arm Contralateral sidebending of spine ◼ Medial rotation of arm Rotation of spine ◼ Lateral rotation of arm Rotation of spine SHOULDER GIRDLE ⦿ Scapulae ⦿ Clavicles ⦿ Sternum ⦿ Provides mobile base for movement of arms CLAVICLE ⦿ Collarbone ⦿ Elongated S shaped bone ⦿ Articulates with Sternum through Manubrium ⦿ Articulates with Scapula through Acromion STERNOCLAVICULAR JOINT STERNOCLAVICULAR JOINT ⦿ Saddle Joint ◼ Between Manubrium and Clavicle ⦿ Movement ◼ Flexion - move forward ◼ Extension - move backward ◼ Elevation - move upward ◼ Depression - move downward ◼ Rotation ⦿ Usually movement happens with scapula Scapula Scapula ● Flat triangular bone ● 3 borders ○ Superior, Medial, Lateral ● 3 angles ○ Superior, Inferior, Lateral ● Processes and Spine ○ Acromion Process, Coracoid Process, Spine of Scapula ● Fossa ○ Supraspinous, Infraspinous, Subscapularis, Glenoid SCAPULA -

Rehabilitation Guidelines for Latarjet/Coracoid Process Transfer Josef K
Rehabilitation Guidelines for Latarjet/Coracoid Process Transfer Josef K. Eichinger, MD Shoulder instability may be caused from congenital deformity, recurrent overuse activity, and/or traumatic dislocation. Surgical stabilization of the glenohumeral joint is indicated after conservative treatment fails and recurrent instability/subluxation continues. A number of different surgical procedures may be indicated in this situation, often divided into soft tissue or bony procedures. Shoulder Instability – Soft Tissue: Surgical reconstruction targeting the glenohumeral joint’s soft tissues for shoulder instability, typically involves labral repairs, the most common being the Bankart repair. A Bankart lesion typically occurs from an anterior-inferior dislocation of the humerus, tearing the labrum from it’s attachment to the glenoid, thereby detaching the inferior gleno-humeral ligament (IGHL). Surgical management of this revolves around labral repair to reattach the IGHL under appropriate tension. This may be accomplished either arthroscopically or through an open approach.1 Most traumatic glenohumeral dislocations may not only cause a Bankart lesion, but may create impression fractures in the postero-superior humeral head termed Hill-Sachs lesions.2 An adverse effect from this procedure includes suturing the capsule too tightly, causing a shortening of the capsule, and thus decreasing the external rotation allowed at the glenohumeral joint. Other complications are extremely rare, but may include axillary nerve damage, subscapularis rupture (seen only in open repairs performed with subscapularis detachment and repair), and recurrent instability. If there is bony deficiency in the glenoid, which represents 20% or more of the antero-inferior glenoid, it is biomechanically impossible to restore the same stability and is therefore more prone to recurrent instability and failure. -
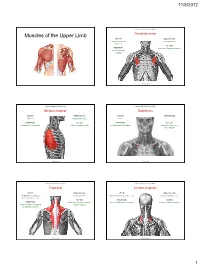
Muscles of the Upper Limb.Pdf
11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles of the Upper Limb Pectoralis minor ORIGIN: INNERVATION: anterior surface of pectoral nerves ribs 3 – 5 ACTION: INSERTION: protracts / depresses scapula coracoid process (scapula) (Anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Serratus anterior Subclavius ORIGIN: INNERVATION: ORIGIN: INNERVATION: ribs 1 - 8 long thoracic nerve rib 1 ---------------- INSERTION: ACTION: INSERTION: ACTION: medial border of scapula rotates scapula laterally inferior surface of scapula stabilizes / depresses pectoral girdle (Lateral view) (anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Trapezius Levator scapulae ORIGIN: INNERVATION: ORIGIN: INNERVATION: occipital bone / spinous accessory nerve transverse processes of C1 – C4 dorsal scapular nerve processes of C7 – T12 ACTION: INSERTION: ACTION: INSERTION: stabilizes / elevates / retracts / upper medial border of scapula elevates / adducts scapula acromion / spine of scapula; rotates scapula lateral third of clavicle (Posterior view) (Posterior view) 1 11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles Moving Arm Rhomboids Pectoralis major (major / minor) ORIGIN: INNERVATION: ORIGIN: INNERVATION: spinous processes of C7 – T5 dorsal scapular nerve sternum / clavicle / ribs 1 – 6 dorsal scapular nerve INSERTION: ACTION: INSERTION: ACTION: medial border of scapula adducts / rotates scapula intertubucular sulcus / greater tubercle flexes / medially rotates / (humerus) adducts -

Applied Anatomy of the Shoulder Girdle
Applied anatomy of the shoulder girdle CHAPTER CONTENTS intra-articular disc, which is sometimes incomplete (menis- Osteoligamentous structures . e66 coid) and is subject to early degeneration. The joint line runs obliquely, from craniolateral to caudomedial (Fig. 2). Acromioclavicular joint . e66 Extra-articular ligaments are important for the stability of Sternoclavicular joint . e66 the joint and to keep the movements of the lateral end of the Scapulothoracic gliding mechanism . e67 clavicle within a certain range. Together they form the roof of Costovertebral joints . e68 the shoulder joint (see Standring, Fig. 46.14). They are the coracoacromial ligament – between the lateral border of the Muscles and their innervation . e69 coracoid process and the acromion – and the coracoclavicular Anterior aspect of the shoulder girdle . e69 ligament. The latter consists of: Posterior aspect of the shoulder girdle . e69 • The trapezoid ligament which runs from the medial Mobility of the shoulder girdle . e70 border of the coracoid process to the trapezoid line at the inferior part of the lateral end of the clavicle. The shoulder girdle forms the connection between the spine, • The conoid ligament which is spanned between the base the thorax and the upper limb. It contains three primary artic- of the coracoid process and the conoid tubercle just ulations, all directly related to the scapula: the acromioclavicu- medial to the trapezoid line. lar joint, the sternoclavicular joint and the scapulothoracic Movements in the acromioclavicular joint are directly related gliding surface (see Putz, Fig. 289). The shoulder girdle acts as to those in the sternoclavicular joint and those of the scapula. a unit: it cannot be functionally separated from the secondary This joint is also discussed inthe online chapter Applied articulations, i.e. -

The Integumentary Skeleton of Tetrapods: Origin, Evolution, and Development Matthew K
J. Anat. (2009) 214, pp441–464 doi: 10.1111/j.1469-7580.2008.01043.x REVIEWBlackwell Publishing Ltd The integumentary skeleton of tetrapods: origin, evolution, and development Matthew K. Vickaryous1 and Jean-Yves Sire2 1Department of Biomedical Sciences, Ontario Veterinary College, University of Guelph, Canada 2Université Pierre et Marie Curie-Paris 6, UMR 7138-Systématique, Adaptation, Evolution, Paris, France Abstract Although often overlooked, the integument of many tetrapods is reinforced by a morphologically and structurally diverse assemblage of skeletal elements. These elements are widely understood to be derivatives of the once all-encompassing dermal skeleton of stem-gnathostomes but most details of their evolution and development remain confused and uncertain. Herein we re-evaluate the tetrapod integumentary skeleton by integrating comparative developmental and tissue structure data. Three types of tetrapod integumentary elements are recognized: (1) osteoderms, common to representatives of most major taxonomic lineages; (2) dermal scales, unique to gymnophionans; and (3) the lamina calcarea, an enigmatic tissue found only in some anurans. As presently understood, all are derivatives of the ancestral cosmoid scale and all originate from scleroblastic neural crest cells. Osteoderms are plesiomorphic for tetrapods but demonstrate considerable lineage-specific variability in size, shape, and tissue structure and composition. While metaplastic ossification often plays a role in osteoderm development, it is not the exclusive mode of skeletogenesis. All osteoderms share a common origin within the dermis (at or adjacent to the stratum superficiale) and are composed primarily (but not exclusively) of osseous tissue. These data support the notion that all osteoderms are derivatives of a neural crest-derived osteogenic cell population (with possible matrix contributions from the overlying epidermis) and share a deep homology associated with the skeletogenic competence of the dermis. -

Development of the Turtle Plastron, the Order-Defining Skeletal Structure
Development of the turtle plastron, the order-defining skeletal structure Ritva Ricea,1, Aki Kallonenb, Judith Cebra-Thomasc,d, and Scott F. Gilberta,c,1 aDevelopmental Biology, Institute of Biotechnology, University of Helsinki, Helsinki 00014, Finland; bDepartment of Physics, University of Helsinki, Helsinki 00014, Finland; cDepartment of Biology, Swarthmore College, Swarthmore, PA 19081; and dDepartment of Biology, Millersville University, Millersville, PA 17551 Edited by Clifford J. Tabin, Harvard Medical School, Boston, MA, and approved March 30, 2016 (received for review January 19, 2016) The dorsal and ventral aspects of the turtle shell, the carapace and the fontanel at the midline of the plastron. Moreover, processes extend plastron, are developmentally different entities. The carapace con- dorsally from the hyoplastron and hypoplastron to form a bridge that tains axial endochondral skeletal elements and exoskeletal dermal connects the plastron with the ribs and the carapace. In some turtles bones. The exoskeletal plastron is found in all extant and extinct (especially ancient lineages), a further set of paired plastron bones, species of crown turtles found to date and is synaptomorphic of the the mesoplastra, lie between the hyoplastra and hypoplastra (7). order Testudines. However, paleontological reconstructed transition Although the anatomy of plastron bones has been known for forms lack a fully developed carapace and show a progression of centuries, and the homology of these bones to the skeletal structures bony elements ancestral to the plastron. To understand the evolu- of other reptilian clades has been debated almost as long (3, 4, 6, 8), tionary development of the plastron, it is essential to know how it has we still know very little about how these intramembranous bones formed. -

Pectoral Girdle Anatomy & Physiology Fundamentals > Skeletal > Skeletal
Pectoral Girdle Anatomy & Physiology Fundamentals > Skeletal > Skeletal Pectoral girdle • Connects the upper limb to the axial skeleton and provides muscle attachment sites for both the arm and back • Comprises the clavicle (collarbone) and the scapula (shoulder blade) Clavicle Anatomy: • Sternal end - flat medial end, articulates with manubrium of sternum • Conoid tubercle - small bony projection where the conoid ligament attaches • Acromion end – rounded lateral end, articulates with acromion of scapula • Costal tuberosity (aka impression) - provides an attachment site for the costoclavicular ligament • Superior surface - smooth • Inferior surface - rough due to muscular attachments Key Points: • Cannot withstand heavy forces; fractures are common and can cause damage to the underlying brachial plexus and associated vessels. • The clavicle is one of the first bones to begin ossification during fetal development, but is the last to complete ossification, during the mid-twenties. • It is the only long bone that ossifies intramembraneously. Scapula Anatomy: • Supraspinous fossa - superior to the spine • Infraspinous fossa - inferior to the spine • Body – triangular surface of scapula • Superior angle – projects superomedially • Inferior angle - projects inferiorly • Lateral angle – projects laterally; includes the glenoid cavity 1 / 2 • Superior border - extends from the glenoid cavity to the superior angle • Lateral (aka axillary) border - extends from the inferior angle to the lateral angle • Medial (aka vertebral) border - extends from -

Coracoid Process Fracture with Acromioclavicular Joint
(aspects of sports medicine) Coracoid Process Fracture With Acromioclavicular Joint Separation in an American Football Player: A Case Report and Literature Review Matthew DiPaola, MD, and Paul Marchetto, MD ABSTRACT 336 collegiate American football to palpation over the AC joint and Coracoid process fractures are rare players had a history of shoulder coracoid process. In addition, he had and few cases have been report- injury, 41% of which were acromio- pain with cross-body adduction of the ed in the orthopedic literature. In clavicular (AC) joint injuries. None symptomatic arm. Anteroposterior this article, we report the case of of these football players had a his- (AP), lateral, and axillary radiographs an American football player with tory of coracoid fracture. of both shoulders were obtained. The a coracoid process fracture in the setting of acromioclavicular sep- Coracoid fractures and ipsilateral AP radiograph showed a nondis- aration and describe incidence, shoulder injuries often occur concur- placed fracture of the coracoid and mechanism of injury, and treatment. rently. Ogawa and colleagues1 found a grade II AC separation (Figures 1, Although rare, coracoid process that 37 of 67 coracoid fractures were 2). Radiographic examination of the fracture should be considered in the associated with ipsilateral AC dis- asymptomatic shoulder confirmed differential diagnosis for shoulder locations. With the incidence of AC that the growth plates in this region pain. Treatment varies according to fracture type. Based on our lit- erature review, we recommend that clinicians initially treat nondisplaced “There is evidence that, of the athletes coracoid fractures nonoperatively. who sustain this injury, football players oracoid process fractures perhaps are at highest risk.” are relatively rare and have been estimated to account for 3% to 13% injury as high as it is in the football were closed.