Light and Emissions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optical Measurements of Atmospheric Aerosols: Aeolian Dust, Secondary Organic Aerosols, and Laser-Induced Incandescence of Soot
Optical Measurements of Atmospheric Aerosols: Aeolian Dust, Secondary Organic Aerosols, and Laser-Induced Incandescence of Soot by Lulu Ma, B. S. A Dissertation In Chemistry Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of Doctor of philosophy Approved Jonathan E. Thompson Chair of Committee Dimitri Pappas Carol L. Korzeniewski Dominick Casadonte Interim Dean of the Graduate School August, 2013 Copyright 2013, Lulu Ma Texas Tech University, Lulu Ma, August 2013 ACKNOWLEDGMENTS I would like to thank my advisor, Dr. Thompson for his guidance and encouragement throughout the past 4 years in Texas Tech University. His profound knowledge, diligence and patience encouraged me and has setup a great example for me in the future. I also thank to Dr. Pappas and Dr. Korzeniewski for their help and for taking time as my committee members. I also would like to thank Dr. Ted Zobeck for his help on soil dust measurements and soil sample collection and also Dr. P. Buseck and his lab for TEM analysis. My group members: Hao Tang, Fang Qian, Kathy Dial, Haley Redmond, Yiyi Wei, Tingting Cao, and Qing Zhang, also helped me a lot in both daily life, and research. At last, I would like to thank my parents and friends for their love and continuous encouragement and support. ii Texas Tech University, Lulu Ma, August 2013 TABLE OF CONTENTS ACKNOWLEDGMENTS……………………………………………………………….ii ABSTRACT ……………………………………………………………………………..vi LIST OF TABLES …………………………………………………………………….viii LIST OF FIGURES……………………………………………………..………………ix LIST OF ABBREVIATIONS ………………………………………….………………xi I. INTRODUCTION ......................................................................................................... 1 1.1 Introduction to Atmospheric Aerosols. ................................................................... 1 1.1.1 Natural Sources ............................................................................................. -

Commentary Kerosene-Based Lighting: an Overlooked Source of Exposure to Household Air Pollution?
Feature: Air quality challenges in low-income settlements Commentary Kerosene-based lighting: an overlooked source of exposure to household air pollution? Ariadna Curto 1,2,3, Cathryn Tonne 1,2,3 1Barcelona Institute for Global Health (ISGlobal), Barcelona, Spain 2Universitat Pompeu Fabra (UPF), Barcelona, Spain 3CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain https://doi.org/10.17159/caj/2020/30/1.8420 Access to affordable, reliable, modern and sustainable energy is prolonged periods, resulting in high levels of inhaled pollutants one of the seventeen Sustainable Development Goals (SDG) set (i.e. more mass inhaled per mass emitted). An experimental by the United Nations for 2030. However, estimates indicate that study in Kenya estimated that night kiosk vendors can inhale progress towards that goal is not on track: 650 million people 1560 µg of fine particles per day emitted by kerosene lamps worldwide are estimated to remain without access to electricity alone (Apple et al., 2010). Relatively few studies have attempted in 2030 (IEA, IRENA, UNSD, WB, WHO, 2019). Nine out of ten of to quantify the contribution of kerosene-based lighting to these people will live in Sub-Saharan Africa (SSA), mostly in rural particle exposure in populations in SSA. We previously reported communities, which face barriers in terms of affordability and that kerosene-based lighting was the strongest determinant supply. of 24-h average and peak personal exposure to black carbon among women living in a semi-rural area of Mozambique (Curto Without access to affordable and reliable clean energy to et al., 2019). Women who used kerosene as the primary source meet daily cooking, lighting, and heating needs, individuals of lighting had 81% and 93% higher average and peak personal rely on inefficient fuels and technologies that give rise to black carbon exposure, respectively, than those using electricity. -
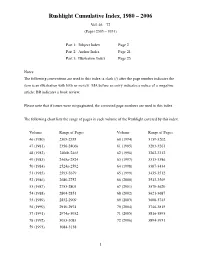
Rushlight Index 1980-2006
Rushlight Cumulative Index, 1980 – 2006 Vol. 46 – 72 (Pages 2305 – 3951) Part 1: Subject Index Page 2 Part 2: Author Index Page 21 Part 3: Illustration Index Page 25 Notes: The following conventions are used in this index: a slash (/) after the page number indicates the item is an illustration with little or no text. MA before an entry indicates a notice of a magazine article; BR indicates a book review. Please note that if issues were mispaginated, the corrected page numbers are used in this index. The following chart lists the range of pages in each volume of the Rushlight covered by this index. Volume Range of Pages Volume Range of Pages 46 (1980) 2305-2355 60 (1994) 3139-3202 47 (1981) 2356-2406a 61 (1995) 3203-3261 48 (1982) 2406b-2465 62 (1996) 3262-3312 49 (1983) 2465a-2524 63 (1997) 3313-3386 50 (1984) 2524a-2592 64 (1998) 3387-3434 51 (1985) 2593-2679 65 (1999) 3435-3512 52 (1986) 2680-2752 66 (2000) 3513-3569 53 (1987) 2753-2803 67 (2001) 3570-3620 54 (1988) 2804-2851 68 (2002) 3621-3687 55 (1989) 2852-2909 69 (2003) 3688-3745 56 (1990) 2910-2974 70 (2004) 3746-3815 57 (1991) 2974a-3032 71 (2005) 3816-3893 58 (1992) 3033-3083 72 (2006) 3894-3951 59 (1993) 3084-3138 1 Rushlight Subject Index Subject Page Andrews' burning fluid vapor lamps 3400-05 Abraham Gesner: Father of Kerosene 2543-47 Andrews patent vapor burner 3359/ Accessories for decorating lamps 2924 Andrews safety lamp, award refused 3774 Acetylene bicycle lamps, sandwich style 3071-79 Andrews, Solomon, 1831 gas generator 3401 Acetylene bicycle lamps, Solar 2993-3004 -

Technology Meets Art: the Wild & Wessel Lamp Factory in Berlin And
António Cota Fevereiro Technology Meets Art: The Wild & Wessel Lamp Factory in Berlin and the Wedgwood Entrepreneurial Model Nineteenth-Century Art Worldwide 19, no. 2 (Autumn 2020) Citation: António Cota Fevereiro, “Technology Meets Art: The Wild & Wessel Lamp Factory in Berlin and the Wedgwood Entrepreneurial Model,” Nineteenth-Century Art Worldwide 19, no. 2 (Autumn 2020), https://doi.org/10.29411/ncaw.2020.19.2.2. Published by: Association of Historians of Nineteenth-Century Art Notes: This PDF is provided for reference purposes only and may not contain all the functionality or features of the original, online publication. License: This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License Creative Commons License. Accessed: October 30 2020 Fevereiro: The Wild & Wessel Lamp Factory in Berlin and the Wedgwood Entrepreneurial Model Nineteenth-Century Art Worldwide 19, no. 2 (Autumn 2020) Technology Meets Art: The Wild & Wessel Lamp Factory in Berlin and the Wedgwood Entrepreneurial Model by António Cota Fevereiro Few domestic conveniences in the long nineteenth century experienced such rapid and constant transformation as lights. By the end of the eighteenth century, candles and traditional oil lamps—which had been in use since antiquity—began to be superseded by a new class of oil-burning lamps that, thanks to a series of improvements, provided considerably more light than any previous form of indoor lighting. Plant oils (Europe) or whale oil (United States) fueled these lamps until, by the middle of the nineteenth century, they were gradually replaced by a petroleum derivative called kerosene. Though kerosene lamps remained popular until well into the twentieth century (and in some places until today), by the late nineteenth century they began to be supplanted by gas and electrical lights. -

Lamp Basics Compiled.Pdf
Lamp Basics Lost-in-Time Museum Lamp Fuel One of the earliest types of lamp fuel was animal tallow, usually rendered from beef or mutton fat. Normally solid at room temperatures, it had to be heated to stay liquid. Lamp designs began to emerge in the late 1700’s using lighter fluids, which could travel vertically up a wick and produce a brighter, more efficient light. Whale oil, already used in candle making, was found to be an excellent oil for this new type of lamp. As the market grew, prices for whale oil soared. Burning fluid and camphene, two substitutes for whale oil, were used as early as the 1830’s, but were dangerous due to their flammability. Their use became obsolete as kerosene became more affordable. Kerosene, first produced in 1846 by distilling cannel coal, was commonly referred to as ‘coal oil’. The word "Kerosene", a registered trademark in 1854, eventually became a generic name used by all refiners. While safer and cleaner than burning fluids and camphene, it was expensive and not affordable to the everyday consumer. Kerosene distilled from petroleum revolutionized the lamp fuel market. As production methods became more efficient in the 1860’s, this new type of kerosene, which produced a brighter and cleaner light, gradually became the fuel source used by the general populace. An improved distribution network for natural gas and electricity, along with the technological advancements they made possible, led to the end of kerosene as a primary fuel source. Kerosene is now mainly used for residential heating and as an additive for insecticides and aviation fuel. -

Tucson Lighting, 1882-1912, with Information on Lighting the Historic Interior
Tucson lighting, 1882-1912, with information on lighting the historic interior Item Type text; Thesis-Reproduction (electronic) Authors Orlando, Catherine Maier Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 28/09/2021 00:48:28 Link to Item http://hdl.handle.net/10150/557708 TUCSON LIGHTING, 1882-1912, WITH INFORMATION ON LIGHTING THE HISTORIC INTERIOR by Catherine Maier Orlando A Thesis Submitted to the Faculty of the SCHOOL OF HOME ECONOMICS In Partial Fulfillment of the Requirements For the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 19 8 1 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfill ment of requirements for an advanced degree at The Univer sity of Arizona and is deposited in the University Library to be made available to borrowers under rules of the Library. Brief quotations from this thesis are allowable without special permission, provided that accurate acknowl edgment of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in his judgment the proposed use of the material is in the inter ests of scholarship. In all other instances, however, permission must be obtained from the author. -

Understanding the Economics Behind Off-Grid Lighting Products for Small Businesses in Kenya
UNDERSTANDING THE ECONOMICS BEHIND OFF-GRID LIGHTING PRODUCTS FOR SMALL BUSINESSES IN KENYA By Kristen Radecsky A Thesis Presented to The Faculty of Humboldt State University In Partial Fulfillment Of the Requirements for the Degree Master of Science In Environmental Systems: Energy, Environment, and Society Option May, 2009 UNDERSTANDING THE ECONOMICS BEHIND OFF-GRID LIGHTING PRODUCTS FOR SMALL BUSINESSES IN KENYA By Kristen Radecsky Approved by the Master's Thesis Committee: Dr. Arne Jacobson, Major Professor Date Dr. Charles Chamberlin, Committee Member Date Dr. Steven Hackett, Committee Member Date Dr. Christopher Dugaw, Graduate Coordinator Date Dr. Chris A. Hopper, Dean for Research and Graduate Studies Date ABSTRACT UNDERSTANDING THE ECONOMICS BEHIND OFF-GRID LIGHTING PRODUCTS FOR SMALL BUSINESSES IN KENYA Kristen Radecsky For illumination, many off-grid communities use lighting products such as candles, kerosene-fueled lamps, or dry cell battery-powered lights. Unfortunately, fuel- based and dry cell powered lighting can be expensive, a health hazard and often provides poor quality light. Manufacturers are currently designing rechargeable lighting products using LED technology as an alternative option for lower-income people. I developed a model to analyze the initial and life cycle costs of 19 off-grid lighting products. With the results, I make design recommendations for manufacturers. The analysis is based on product prices, laboratory measurements of product performance, and data about lighting cost and use patterns for small, off-grid businesses in Kenya. The field data were collected by Arne Jacobson, Maina Mumbi, Peter Johnstone and me during 2008. My results indicate that the economics of off-grid lighting using electric lamps depends on the charging mode. -

USER MANUAL / INSTRUCTIONS Rapid 829/500CP Multi Fuel Lantern
\ USER MANUAL / INSTRUCTIONS Rapid 829/500CP Multi Fuel Lantern BriteLyt Rapid 829/500CPREV 4.3 0801_13 1 Rapid 829/500CP Multi-Fuel Lantern Lantern Master Diagram 2 Spare Parts/Tool Kits 3 Lantern Parts Diagram 4 Warranty / Tech. Support 6 Prior to starting your BriteLyt 7 Step by Step Lighting Instructions 8 Shutdown Procedure 12 Storage Procedure 12 Tips, Troubleshooting / Warranty Reg. 13 EZ-Pump Instructions 14 EZ-Cook Instructions 15 Mutli-Fuel Operation 16 For a video tutorial on how to use your lantern or troubleshooting suggestions visit: www.BriteLyt.com/howto Welcome to the BriteLyt Family! Congratulations, on your choice of the new BriteLyt, Multi-Fuel Lantern! Many years of passion and lantern know-how have gone into creating the most functional pressurized liquid-fuel lantern ever! On behalf of all of us at BriteLyt congratulations and we hope you enjoy your new lantern for many years to come! IMPORTANT: Read this manual in its entirety. BriteLyt assumes no responsibility for the use of your lantern outside of normal operating conditions. It is very important that you understand all topics covered in this manual be- fore attempting operation or fuel use of any kind. Some sections of this manual reflect optional acces- sories that are not shipped standard with every lan- tern. To purchase accessories, visit your local retailer or www.britelyt.com. BriteLyt Rapid 829/500CP 1 Lantern Master Diagram 1) Hood/Cover 8) Carburetor 2) Regulator Screw 9) Rapid Pre-Heater 3) Hood Inner Casing 10) Fuel Dial 4) Non-Ceramic Burner Assembly 11) Bleeder Screw 5) Handle/Reflector Screws 12) Hand Pump 6) Needle and Spray Nozzle 13) Pressure Gauge 7) Preheat Cup 14) Fuel Tank 2 BriteLyt Rapid 829/500CP Parts Kits Your BriteLyt lantern is shipped with a parts/tools kit packet. -

50 Ways Kerosene Lighting
50 ways to end kerosene lighting May 2009 ACKNOWLEDGEMENTS The authors wish to thank the Department of the Environment, Water, Heritage and the Arts (DEWHA - formerly the Australian Greenhouse Office), the Renewable Energy and Energy Efficiency Partnership (REEEP), and the many pioneers who have developed the technologies listed within, to aid in the fight against energy poverty. Many thanks also go to our family, friends, investors and supporters, who continue to provide fantastic support during our mission to help 1 million people gain access to clean, efficient lighting and energy. DISCLAIMER The authors have used publicly available website reference information and use of photos, and thank the authors of each and every of these images. The authors have made their best estimates of current expected retail prices of these technologies from publicly available information, but have not contacted the suppliers of each product to confirm pricing. The technical descriptions have also created by the authors, in an attempt to accurately portray the system components, performance, sizing and pricing, but this data should be strictly taken as a guide only. Suppliers of these technologies should be contacted to obtain current and accurate technical specifications and prices. The reader should not use this guide as the sole basis of commercial decision making, or even the ranking of products. Product information was not verified, and Barefoot Power nor any of its employees make any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of the information provided or represents that its use would not infringe privately owned rights. -

Chapter 2 Incandescent Light Bulb
Lamp Contents 1 Lamp (electrical component) 1 1.1 Types ................................................. 1 1.2 Uses other than illumination ...................................... 2 1.3 Lamp circuit symbols ......................................... 2 1.4 See also ................................................ 2 1.5 References ............................................... 2 2 Incandescent light bulb 3 2.1 History ................................................. 3 2.1.1 Early pre-commercial research ................................ 4 2.1.2 Commercialization ...................................... 5 2.2 Tungsten bulbs ............................................. 6 2.3 Efficacy, efficiency, and environmental impact ............................ 8 2.3.1 Cost of lighting ........................................ 9 2.3.2 Measures to ban use ...................................... 9 2.3.3 Efforts to improve efficiency ................................. 9 2.4 Construction .............................................. 10 2.4.1 Gas fill ............................................ 10 2.5 Manufacturing ............................................. 11 2.6 Filament ................................................ 12 2.6.1 Coiled coil filament ...................................... 12 2.6.2 Reducing filament evaporation ................................ 12 2.6.3 Bulb blackening ........................................ 13 2.6.4 Halogen lamps ........................................ 13 2.6.5 Incandescent arc lamps .................................... 14 2.7 Electrical -

The Pollutant Exposure Impact of Solar Lighting: Results from a Pilot Experiment in Rural Kenya
©Patrick Bentley/SolarAid The Pollutant Exposure Impact of Solar Lighting: Results from a pilot experiment in rural Kenya Nicholas Lam, Michael Bates, Kat Harrison, Godfrey Muhwezi, Fred Isabirye, Ilse Ruiz-Mercado, Evans Amukoye, Tom Mokaya, Margaret Wambua, Ian Bailey April 2017 1 Introduction A small study was carried out in Busia County, Kenya, to quantify changes in exposure to fine particulate matter (PM2.5) and carbon monoxide (CO) associated with introducing solar lamps into households using kerosene lamps as their primary lighting source. Popularity and sales of solar lighting have been increasing rapidly in developing countries, particularly in sub-Saharan Africa and South Asia. It has been suggested that solar lighting devices may provide a near-term alternative to polluting kerosene lamps, and other forms of fuel-based lighting, in areas with limited access to electricity. Whether there are health benefits to families using solar lamps rather than kerosene lamps has not been scientifically investigated. Evidence has been increasing, however, that uses of kerosene lighting and cooking devices in households are associated with serious health effects, particularly involving the lungs, but possibly also the eyes and to unborn babies. Evidence also suggests that these effects are caused by exposure to the pollutants emitted by the kerosene devices when they are operated. The level of pollution to which an individual is exposed is often used as an indicator of health risk. The size of exposure changes is a critical input in the design of any health study intended to directly measure health impacts. Such health-focused studies typically require large sample sizes. -

Lighting Technologies
Lighting Technologies Information & Knowledge Management Technical Information Lighting Technologies J Energy / Environment (E) F Water / Sanitation (W) F Agriculture (A) F Foodprocessing (F) F Manufacturing (M) This module is available in: Dishna Schwarz J English (e) Elmar Dimpl F French (f) George C. Bandlamudi F German (g) F Spanish (s) F Other(s): ........................... September 2005 File: E023E_Lighting Technologies.pdf 1 Introduction The rural poor in most of the developing radio waves. But, the human eye is countries still lack access to energy and sensitive only to radiation with simultaneously are incapacitated to afford wavelengths in the range of 0.38 to 0.76 good lighting. It is worthwhile to examine micrometre, which we call ‘the visible and consider lighting methods that would spectrum’. Radiation is the cause and the be economical in energy and cost, to be visible light is the result. It takes a certain successfully disseminated amongst this amount of energy to produce a given target group. amount of light. Light of one fixed wavelength is termed as ‘monochromatic’ 2 Some facts about Light light, characterised by its corresponding colour at that wavelength. For instance, if Light is a form of energy, which can be a lamp is producing only radiation of 0.555 defined both as i) an electro-magnetic micrometre, then the resulting light is radiation and ii) a particle, as illustrated in monochromatic, with its yellow-green Fig.1. colour. Sensitivity of the human eye is 100% at a wavelength of 0.555 micrometre. The electromagnetic wave spectrum is illustrated in Fig.2. Light, as particles or packets of energy, more popularly known as ‘photons’.