High-Resolution Mapping of Transcriptional Dynamics Across Tissue Development Reveals a Stable Mrna–Trna Interface
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
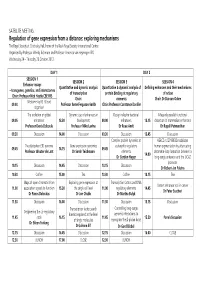
SM08 Programme
SATELLITE MEETING Regulation of gene expression from a distance: exploring mechanisms The Royal Society at Chicheley Hall, home of the Kavli Royal Society International Centre Organised by Professor Wendy Bickmore and Professor Veronica van Heyningen FRS Wednesday 24 – Thursday 25 October 2012 DAY 1 DAY 2 SESSION 1 SESSION 2 SESSION 3 SESSION 4 Enhancer assays Quantitative and dynamic analysis Quantitative & dynamic analysis of Defining enhancers and their mechanisms – transgenes, genetics, and interactomes of transcription protein binding at regulatory of action Chair: Professor Nick Hastie CBE FRS Chair: elements Chair: Dr Duncan Odom Welcome by RS & lead 09.00 Professor Anne Ferguson-Smith Chair: Professor Constance Bonifer organiser The evolution of global Dynamic use of enhancers in Design rules for bacterial Massively parallel functional 09.05 enhancers 13.30 development 09.00 enhancers 13.15 dissection of mammalian enhancers Professor Denis Duboule Professor Mike Levine Dr Roee Amit Dr Rupali Patwardhan 09.30 Discussion 14.00 Discussion 09.30 Discussion 13.45 Discussion Complex protein dynamics at HERC2 rs12913832 modulates The pluripotent 3D genome Gene expression genomics eukaryotic regulatory human pigmentation by attenuating 09.45 14.15 09.45 Professor Wouter de Laat Dr Sarah Teichmann elements chromatin-loop formation between a 14.00 Dr Gordon Hager long-range enhancer and the OCA2 promoter 10.15 Discussion 14.45 Discussion 10.15 Discussion Dr Robert-Jan Palstra 10.30 Coffee 15.00 Tea 10.30 Coffee 14.15 Tea Maps of open chromatin -

DKFZ Postdoctoral Fellowships 2021 Hosting Group Information For
DKFZ Postdoctoral Fellowships 2021 Hosting group information for applicants Name of DKFZ research division/group: Division of Regulatory Genomics and Cancer Evolution (B270) Contact person: Duncan Odom Group homepage: https://www.dkfz.de/en/regulatorische-genomik/index.php Please visit our website for further information on our research and recent publications. RESEARCH PROFILE AND PROJECT TOPICS: Dr Odom’s laboratory studies how genetic sequence information shapes the cell's DNA regulatory landscape and thus the trajectory of cancer genome evolution. Our long-standing interest in interspecies analysis of matched functional genomic data has revealed the extensive and rapid turn-over of tissue-specific transcription factor binding, insulator elements, polymerase occupancies, and enhancer activities during organismal evolution. My laboratory has begun exploiting single-cell RNA-sequencing and large-scale whole genome sequencing in understanding molecular and cancer genome evolution. Recent high-profile studies from the Odom lab used single-cell transcriptional analysis to conclusively demonstrate that ageing results in substantial increases in cell-to-cell transcriptional variability and exploited chemical carcinogenesis in mouse liver to reveal how the persistence of DNA lesions profoundly shapes tumour genomes. First, we are currently interviewing for experimentally focused postdoctoral fellows to spearhead multiple diverse and interdisciplinary collaborations in single-cell analysis, functional genomics, and spatial transcriptomics with Gerstung, Stegle, Hoefer, and Goncalves laboratories at DKFZ. Such an appointment would offer unusual opportunities for an experimentally-focused postdoc to learn and deploy bioinformatics tools in collaboration with one or more world-leading computational biology laboratories. Second, we are seeking a postdoctoral researcher to spearhead a collaboration with Edith Heard's group at EMBL, focused on how X-chromosome dynamics can shape disease susceptibility in older individuals. -

Paterson Institute for Cancer Research Scientific Report 2008 Contents
paterson institute for cancer research scientific report 2008 cover images: Main image supplied by Karim Labib and Alberto sanchez-Diaz (cell cycle Group). Budding yeast cells lacking the inn1 protein are unable to complete cytokinesis. these cells express a fusion of a green fluorescent protein to a marker of the plasma membrane, and have red fluorescent proteins attached to components of the spindle poles and actomyosin ring (sanchez-Diaz et al., nature cell Biology 2008; 10: 395). Additional images: front cover image supplied by Helen rushton, simon Woodcock and Angeliki Malliri (cell signalling Group). the image is of a mitotic spindle in fixed MDcK (Madin-Darby canine kidney) epithelial cells, which have been stained with an anti-beta tubulin antibody (green), DApi (blue) and an anti-centromere antibody (crest, red) which recognises the kinetochores of the chromosomes. the image was taken on the spinning disk confocal microscope using a 150 x lens. rear cover image supplied by Andrei ivanov and tim illidge (targeted therapy Group). Visualisation of tubulin (green) and quadripolar mitosis (DnA stained with DApi), Burkitt’s lymphoma namalwa cell after 10 Gy irradiation. issn 1740-4525 copyright 2008 © cancer research UK Paterson Institute for Cancer Research Scientific Report 2008 Contents 4 Director’s Introduction Researchers’ pages – Paterson Institute for Cancer Research 8 Crispin Miller Applied Computational Biology and Bioinformatics 10 Geoff Margison Carcinogenesis 12 Karim Labib Cell Cycle 14 Iain Hagan Cell Division 16 Nic Jones -

Register of Declared Private, Professional Commercial and Other Interests
REGISTER OF DECLARED PRIVATE, PROFESSIONAL COMMERCIAL AND OTHER INTERESTS Please refer to the attached guidance notes before completing this register entry. In addition to guidance on each section, examples of information required are also provided. Where you have no relevant interests in the relevant category, please enter ‘none’ in the register entry. Name: Michaela Frye Please list all MRC bodies you are a member of: E.g. Council, Strategy Board, Research Board, Expert Panel etc and your position (e.g. chair, member). Regenerative Medicine Research Committee member Main form of employment: Name of University and Department or other employing body (include location), and your position. University of Cambridge, Department of Genetics, Cancer Research UK Senior Fellow Research group/department web page: Provide a link to any relevant web pages for your research group or individual page on your organisation’s web site. http://www.stemcells.cam.ac.uk/research/affiliates/dr-frye http://www.gen.cam.ac.uk/research-groups/frye Please give details of any potential conflicts of interests arising out of the following: 1. Personal Remuneration: Including employment, pensions, consultancies, directorships, honoraria. See section 1 for further guidance. None 2. Shareholdings and Financial Interests in companies: Include the names of companies involved in medical/biomedical research, pharmaceuticals, biotechnology, healthcare provision and related fields where shareholdings or other financial interests. See section 2 for thresholds and further guidance. None 3. Research Income during current session (financial year) : Declare all research income from bodies supported by the MRC and research income from other sources above the limit of £50k per grant for the year. -

Transcription Factor Binding Cells and Their Progeny
RESEARCH HIGHLIGHTS knock-in alleles at the Lgr6 locus, allowing them to visualize Lgr6+ Transcription factor binding cells and their progeny. Shortly after birth, Lgr6 expression in the skin becomes restricted, eventually marking a unique population located at Most transcription factor binding events differ between closely the central isthmus just above the bulge region in resting hair follicles. related species despite conservation of functional targets. Now, Paul Flicek and Duncan Odom and colleagues report an Through a series of lineage-tracing studies in adult mice, they showed + evolutionary comparison of transcription factor CEBPA binding that the progeny of Lgr6 cells contribute not only to hair follicles but among five vertebrate species (Science published online, also to sebaceous glands and interfollicular epidermis. They also showed + doi:10.1126/science.1186176, 8 April 2010). The authors that transplanted Lgr6 stem cells could reconstitute fully formed hair compared genome-wide binding of CEBPA as determined by follicles and contribute to all three lineages in host skin. Finally, they + ChIP-sequencing in livers of human, mouse, dog, short-tailed showed that Lgr6 cells contribute to wound healing. These findings opossum and chicken. The CEBPA binding motif was virtually identify Lgr6 as a marker of a distinct epidermal stem cell population identical between the five species, but most binding events contributing to all three skin lineages. KV were species-specific. Only 10% to 22% of binding events were shared between any two placental mammals, and there was even greater divergence of binding events in opossum and chicken. Identifying nonobvious phenologs Only 35 binding events were shared by all five species. -

Decoupling of Evolutionary Changes in Transcription Factor Binding and Gene Expression in Mammals
Downloaded from genome.cshlp.org on April 13, 2016 - Published by Cold Spring Harbor Laboratory Press Research Decoupling of evolutionary changes in transcription factor binding and gene expression in mammals Emily S. Wong,1 David Thybert,1 Bianca M. Schmitt,2 Klara Stefflova,2,4 Duncan T. Odom,2,3 and Paul Flicek1,3 1European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1SD, United Kingdom; 2University of Cambridge, Cancer Research UK - Cambridge Institute, Li Ka Shing Centre, Cambridge, CB2 0RE, United Kingdom; 3Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1SA, United Kingdom To understand the evolutionary dynamics between transcription factor (TF) binding and gene expression in mammals, we compared transcriptional output and the binding intensities for three tissue-specific TFs in livers from four closely related mouse species. For each transcription factor, TF-dependent genes and the TF binding sites most likely to influence mRNA expression were identified by comparing mRNA expression levels between wild-type and TF knockout mice. Independent evolution was observed genome-wide between the rate of change in TF binding and the rate of change in mRNA ex- pression across taxa, with the exception of a small number of TF-dependent genes. We also found that binding intensities are preferentially conserved near genes whose expression is dependent on the TF, and the conservation is shared among binding peaks in close proximity to each other near the TSS. Expression of TF-dependent genes typically showed an increased sensitivity to changes in binding levels as measured by mRNA abundance. -

Extensive Compensatory Cis-Trans Regulation in the Evolution of Mouse Gene Expression
Downloaded from genome.cshlp.org on September 25, 2021 - Published by Cold Spring Harbor Laboratory Press Research Extensive compensatory cis-trans regulation in the evolution of mouse gene expression Angela Goncalves,1,5 Sarah Leigh-Brown,2,3,5 David Thybert,1,6 Klara Stefflova,3,6 Ernest Turro,2,3 Paul Flicek,1,4 Alvis Brazma,1 Duncan T. Odom,2,3,4,7 and John C. Marioni1,7 1European Bioinformatics Institute (EMBL-EBI), Wellcome Trust Genome Campus, Hinxton, Cambridge CB10 1SD, United Kingdom; 2Department of Oncology, University of Cambridge, Hills Road, Cambridge CB2 0XZ, United Kingdom; 3Cancer Research UK, Cambridge Research Institute, Robinson Way, Cambridge CB2 0RE, United Kingdom; 4Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge CB10 1SA, United Kingdom Gene expression levels are thought to diverge primarily via regulatory mutations in trans within species, and in cis between species. To test this hypothesis in mammals we used RNA-sequencing to measure gene expression divergence between C57BL/6J and CAST/EiJ mouse strains and allele-specific expression in their F1 progeny. We identified 535 genes with parent-of-origin specific expression patterns, although few of these showed full allelic silencing. This suggests that the number of imprinted genes in a typical mouse somatic tissue is relatively small. In the set of nonimprinted genes, 32% showed evidence of divergent expression between the two strains. Of these, 2% could be attributed purely to variants acting in trans, while 43% were attributable only to variants acting in cis. The genes with expression divergence driven by changes in trans showed significantly higher sequence constraint than genes where the divergence was explained by variants acting in cis. -

Decoupling of Evolutionary Changes in Transcription Factor Binding and 10 Gene Expression in Mammals
Downloaded from genome.cshlp.org on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press 5 Decoupling of evolutionary changes in transcription factor binding and 10 gene expression in mammals 15 20 Emily S. Wong1, David Thybert1, Bianca M. Schmitt2, Klara Stefflova2,4, Duncan T. Odom*2,3, Paul Flicek*1,3 25 * Corresponding authors: DTO ([email protected]); PF ([email protected]) 30 1 European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1SD, UK. 2 University of Cambridge, Cancer Research UK - Cambridge Institute, Li Ka Shing Centre, 35 Cambridge, CB2 0RE, UK. 3 Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1SA, UK. 4 Present address: Caltech Division of Biology and Biological Engineering, 1200 E California Blvd, Pasadena, CA, USA, 91125 40 Running title: Evolution of TF binding and gene expression Keywords: gene regulation, transcriptional evolution Downloaded from genome.cshlp.org on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press ABSTRACT To understand the evolutionary dynamics between transcription factor (TF) binding and gene expression in mammals, we compared transcriptional output and the binding intensities for 5 three tissue-specific TFs in livers from four closely related mouse species. For each transcription factor, TF dependent genes and the TF binding sites most likely to influence mRNA expression were identified by comparing mRNA expression levels between wildtype and TF knockout mice. Independent evolution was observed genome-wide between the rate of change in TF binding and the rate of change in mRNA expression across taxa, with the 10 exception of a small number of TF dependent genes. -

Interplay of Cis and Trans Mechanisms Driving Transcription Factor Binding and Gene Expression Evolution
bioRxiv preprint doi: https://doi.org/10.1101/059873; this version posted July 29, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Interplay of cis and trans mechanisms driving transcription factor binding and gene expression evolution Emily S Wong1*, Bianca M Schmitt2*, Anastasiya Kazachenka3, David Thybert1, Aisling Redmond2, Frances Connor2, Tim F. Rayner2, Christine Feig2, Anne C. Ferguson-Smith3, John C Marioni1,2, Duncan T Odom2,4#, Paul Flicek1,4# * These authors contributed equally # Co-corresponding authors: DTO ([email protected]), PF ([email protected]). 1 European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Genome Campus, Hinxton, Cambridge, CB10 1SD, UK. 2 University of Cambridge, Cancer Research UK - Cambridge Institute, Li Ka Shing Centre, Cambridge, CB2 0RE, UK. 3 University of Cambridge, Department of Genetics, Cambridge, CB2 3EH, UK. 4 Wellcome Trust Sanger Institute, Wellcome Genome Campus, Hinxton, Cambridge, CB10 1SA, UK. 1 bioRxiv preprint doi: https://doi.org/10.1101/059873; this version posted July 29, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Noncoding regulatory variants play a central role in the genetics of human diseases and in evolution. Here we measure allele-specific transcription factor binding occupancy of three 5 liver-specific transcription factors between crosses of two inbred mouse strains to elucidate the regulatory mechanisms underlying transcription factor binding variations in mammals. -

Tumours at Cellular Resolution
Cancer Research UK Cambridge Institute Annual International Symposium Unanswered Questions: Tumours at Cellular Resolution 4 – 5 March 2016 Programme Programme CRUK CI Annual International Symposium 2016 11 Friday 4 March 9.30-9.35 Welcome from Simon Tavaré Session 1 Tissue architecture: imaging and bioengineering Chair: John Marioni Programme Cancer Research UK Cambridge Institute and EBI 9.35-9.40 Session introduction by John Marioni 9.40-10.10 Mikala Egeblad Cold Spring Harbor Laboratory Caught in the act: cancer cell activities revealed by imaging in live tissues 10.10-10.40 Lucas Pelkmans University of Zurich Control of transcript variability in single mammalian cells 10.40-10.55 Selected talk from submitted abstracts: to be confirmed 10.55-11.25 Coffee/tea 11.25-11.55 AlisonDRAFT McGuigan University of Toronto TRACER: An engineered tumour for exploring cellular phenotype and microenvironment in hypoxic gradients 11.55-12.25 Itai Yanai Technion - Israel Institute of Technology Deciphering the expression of all genes in all cells throughout the development of the nematode C. elegans 12.25-13.10 "Unanswered questions" panel discussion introduced by John Marioni, Cancer Research UK Cambridge Institute and EBI 13.10-15.00 Lunch (and poster session) 12 CRUK CI Annual International Symposium 2016 Session 2 Approaches to understanding tumours at cellular resolution Programme Chair: Simon Tavaré Cancer Research UK Cambridge Institute 15.00-15.05 Session introduction by Simon Tavaré 15.05-15.35 Rahul Satija New York University Learning the -

Waves of Retrotransposon Expansion Remodel Genome Organization and CTCF Binding in Multiple Mammalian Lineages
Waves of Retrotransposon Expansion Remodel Genome Organization and CTCF Binding in Multiple Mammalian Lineages Dominic Schmidt,1,2,6 Petra C. Schwalie,3,6 Michael D. Wilson,1,2 Benoit Ballester,3 Aˆ ngela Gonc¸alves,3 Claudia Kutter,1,2 Gordon D. Brown,1,2 Aileen Marshall,1,5 Paul Flicek,3,4,* and Duncan T. Odom1,2,4,* 1Cancer Research UK, Cambridge Research Institute, Li Ka Shing Centre, Robinson Way, Cambridge CB2 0RE, UK 2Department of Oncology, Hutchison/MRC Research Centre, University of Cambridge, Hills Road, Cambridge CB2 0XZ, UK 3European Bioinformatics Institute (EMBL-EBI), Wellcome Trust Genome Campus, Hinxton, Cambridge CB10 1SD, UK 4Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge CB10 1SA, UK 5Cambridge Hepatobiliary Service, Addenbrooke’s Hospital, Hills Road, Cambridge CB2 2QQ, UK 6These authors contributed equally to this work *Correspondence: fl[email protected] (P.F.), [email protected] (D.T.O.) DOI 10.1016/j.cell.2011.11.058 SUMMARY from fly to human (Burcin et al., 1997; Klenova et al., 1993; Moon et al., 2005). Originally identified as a transcriptional regu- CTCF-binding locations represent regulatory se- lator for the c-myc oncogene (Baniahmad et al., 1990; Filippova quences that are highly constrained over the course et al., 1996; Lobanenkov et al., 1990), CTCF remains the only of evolution. To gain insight into how these DNA identified sequence-specific DNA-binding protein that helps elements are conserved and spread through the establish vertebrate insulators (Bell et al., 1999). Additionally, genome, we defined the full spectrum of CTCF- CTCF has been implicated in transcriptional activation, repres- binding sites, including a 33/34-mer motif, and iden- sion, silencing, and imprinting of genes (Awad et al., 1999; Burcin et al., 1997; Filippova et al., 1996; Klenova et al., 1993; Lobanen- tified over five thousand highly conserved, robust, kov et al., 1990). -

Wednesday 26 OCTOBER Thursday 27 OCTOBER
Wednesday 26 OCTOBER Thursday 27 OCTOBER 11:00 Maria Leptin Welcome address 08:25 Bus from Villa Toskana Hotel 08:30 Bus from ISG Hotel SESSION 1 Suzanne Eaton CHAIR Max Planck Institute of Molecular Cell Biology & Genetics, Dresden, DE 08:30 – 09:00 COFFEE BREAK EMBL OPERON FOYER 11:10 Helder Maiato Cracking the code of mitosis University of Porto, PT SESSION 5 Michael Way CHAIR The Francis Crick Institute, London, GB 11:30 Eugene Myers A forward-looking vision for microscopy and image analysis 09:00 Buzz Baum Cell division: its origins and mechanics Max Planck Institute of Molecular Cell Biology & Genetics, Dresden, DE University College London, GB 11:50 Adam Antebi Convergent mechanisms of longevity 09:20 Pekka Lappalainen Assembly of mechanosensitive actomyosin bundles in migrating cells Max Planck Institute for Biology of Ageing, Cologne, DE University of Helsinki, FI 12:10 Uwe Sauer Coordination of metabolism 09:40 Carsten Janke The tubulin code – generating signals to control microtubule function ETH Zurich, CH Institut Curie, Orsay, FR 12:30 Ehud Gazit Metabolite amyloids: a new paradigm for inborn error of metabolism 10:00 Carl-Philipp Heisenberg Cell and tissue mechanics in zebrafish gastrulation disorders Institute of Science and Technology Austria, Klosterneuburg, AT Tel Aviv University, IL 10:20 Erik Sahai Modelling lethal cancer phenotypes 12:50 – 13:20 LUNCH EMBL OPERON FOYER The Francis Crick Institute, London, GB SESSION 2 Javier Paz-Ares CHAIR Universidad Autónoma de Madrid, ES 10:40 – 11:00 COFFEE BREAK EMBL OPERON FOYER