Repeat Associated Mechanisms of Genome Evolution and Function Revealed by the Mus Caroli and Mus Pahari Genomes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
SM08 Programme
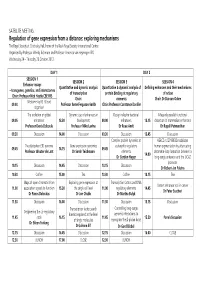
SATELLITE MEETING Regulation of gene expression from a distance: exploring mechanisms The Royal Society at Chicheley Hall, home of the Kavli Royal Society International Centre Organised by Professor Wendy Bickmore and Professor Veronica van Heyningen FRS Wednesday 24 – Thursday 25 October 2012 DAY 1 DAY 2 SESSION 1 SESSION 2 SESSION 3 SESSION 4 Enhancer assays Quantitative and dynamic analysis Quantitative & dynamic analysis of Defining enhancers and their mechanisms – transgenes, genetics, and interactomes of transcription protein binding at regulatory of action Chair: Professor Nick Hastie CBE FRS Chair: elements Chair: Dr Duncan Odom Welcome by RS & lead 09.00 Professor Anne Ferguson-Smith Chair: Professor Constance Bonifer organiser The evolution of global Dynamic use of enhancers in Design rules for bacterial Massively parallel functional 09.05 enhancers 13.30 development 09.00 enhancers 13.15 dissection of mammalian enhancers Professor Denis Duboule Professor Mike Levine Dr Roee Amit Dr Rupali Patwardhan 09.30 Discussion 14.00 Discussion 09.30 Discussion 13.45 Discussion Complex protein dynamics at HERC2 rs12913832 modulates The pluripotent 3D genome Gene expression genomics eukaryotic regulatory human pigmentation by attenuating 09.45 14.15 09.45 Professor Wouter de Laat Dr Sarah Teichmann elements chromatin-loop formation between a 14.00 Dr Gordon Hager long-range enhancer and the OCA2 promoter 10.15 Discussion 14.45 Discussion 10.15 Discussion Dr Robert-Jan Palstra 10.30 Coffee 15.00 Tea 10.30 Coffee 14.15 Tea Maps of open chromatin -

Citizen Participation in the American Federal System"
For sale by the Superintendent of Documents, U.S. Government Printing ORice, Washington, D.C.20402 Preface iii In its 1976 legislation reenacting the General views are included as an appendix to this report, Revenue Sharing program for a second three-year along with a staff summary and evaluation of all period, Congress requested ACIR to prepare the comments received. The comment period several studies of fiscal decisionmaking in the extended from early December 1978 through mid- American federal system of government. Citizen March 1979. Many comments were received, participation, the topic of one of those studies, is some of considerable length and detail, and they the subject of this report. were helpful in revising our report. The number The draft report was prepared by the staff of recommendation options presented to the under its regular procedures which provide for Commission was doubled, and numerous critiques by the governmental research com- refinements in the background chapters were munity and others having special knowledge of made as a result of this consultation process. All the topic. Because of the nature of this topic, the comments received by the first of March were Commission also held a hearing on the draft fully reflected in information presented to the report at its regular meeting on December 7,1978. Commission prior to its action on this report. Following this hearing, the Commission deter- The Commission adopted the recommen- mined that opportunities should be provided for dations in this report and authorized its printing a broader range of views to be submitted on Friday, March 23, 1979. -

Augustine and the Art of Ruling in the Carolingian Imperial Period
Augustine and the Art of Ruling in the Carolingian Imperial Period This volume is an investigation of how Augustine was received in the Carolingian period, and the elements of his thought which had an impact on Carolingian ideas of ‘state’, rulership and ethics. It focuses on Alcuin of York and Hincmar of Rheims, authors and political advisers to Charlemagne and to Charles the Bald, respectively. It examines how they used Augustinian political thought and ethics, as manifested in the De civitate Dei, to give more weight to their advice. A comparative approach sheds light on the differences between Charlemagne’s reign and that of his grandson. It scrutinizes Alcuin’s and Hincmar’s discussions of empire, rulership and the moral conduct of political agents during which both drew on the De civitate Dei, although each came away with a different understanding. By means of a philological–historical approach, the book offers a deeper reading and treats the Latin texts as political discourses defined by content and language. Sophia Moesch is currently an SNSF-funded postdoctoral fellow at the University of Oxford, working on a project entitled ‘Developing Principles of Good Govern- ance: Latin and Greek Political Advice during the Carolingian and Macedonian Reforms’. She completed her PhD in History at King’s College London. Augustine and the Art of Ruling in the Carolingian Imperial Period Political Discourse in Alcuin of York and Hincmar of Rheims Sophia Moesch First published 2020 by Routledge 2 Park Square, Milton Park, Abingdon, Oxon OX14 4RN and by Routledge 52 Vanderbilt Avenue, New York, NY 10017 Routledge is an imprint of the Taylor & Francis Group, an informa business Published with the support of the Swiss National Science Foundation. -

The Arl<Ansas Family Historian
The Arl<ansas Family Historian Volume 22, No.4, December 1984 Note: There is a surname index for Vol. 22 at the end of issue No.4. THE ARKANSAS FAMILY HISTORIAN VOlUME 22 NO. If. DEtEMBER 191. PuLli.hed. Quarterly By ARKANSAS GENEALOGICAL SOCIETY ARKANSAS GENEAlOOlCAL SOCIEl'Y 1962 The following is quoted fran the Constitution of the Arkansas Genealogi cal 8ociety, approved and a<bpted in 1965: ARTICIE II -- PIJRl?OOE The purpose of the Arkansas Genealogical 80ciey includes the following aspects: (a) to bring together interested persons for discussion and interchange of infonm.tion in thE< field of genealogy. (b) to foster the study of methods of research in genealogy and family history. (c) to assist individual manbers and others in research in genealogy and family history. (d) to issue appropriate publications. (e) to collect and preserve appropriate source material. (f) to assist libraries in developing adequate genealogical collections . (g) to encourage the establisbnent of local chapters of this society within the state. (h) to seek by all appropriate means the generation of greater interest in and suP:lX'rt of genealogical study by the public as a whole. '" '" '" * * 'lliE ARKANSAS FAMILY HISTORIAN is the official publication of the Arkansas Genealogical Society. It is published quarterly by the 80ciety and is entered in themailsunderThirdC1assPermitlb.509atEbtSprings.AR. M:l!Ibership rate is $12.00 per calendar year. Fbur issues constitute one year's rreJbership. Manberships may he entered by submission of dues and enrollment data to Ms. Margaret Hubbard, 1207 Shady Grove !:bad. Hot Springs, AR 71901. Nei ther the Arkansas Genealogical 8::lciety. -

Crystal Reports Activex Designer
PrimeTime Timing - Contractor License Hy-Tek's MEET MANAGER 3:44 PM 4/6/2019 Page 1 Spec Towns Invitational - 4/5/2019 to 4/6/2019 Spec Towns Track University of Georgia - Athens, GA Results ---Jonae Cook FR Arkansas Sta DNS 1.2 Girls 100 Meter Dash ---Osereme Erewele FR Arkansas Sta DNS 1.2 NameYr School Finals ---Karel Ziketh Oxy Athletics DNS 1.2 Finals 1Chloe Love Unattached 15.12 -0.6 Women 400 Meter Dash 2Violet Patton Unattached 15.12 -0.8 Collegiate: 49.71 C4/23/2016 Courtney Okolo 3Atuyah Thompson Unattached 15.32 -0.8 NameYr School Finals 4Mackenzie Moore Unattached 15.50 -0.6 Finals 5Trahziha Brightwell Unattached 15.56 -0.6 1Aliyah Abrams JR South Caroli 52.30 6Roane Hondon Unattached 15.60 -0.8 2Micaiah Ransby JR Georgia 53.71 7Sharniece Gonzalez Unattached 15.80 -0.8 3Orianna Shaw SO Ole Miss 55.24 8Layla White Unattached 16.50 -0.6 4Tatyana Mills SR South Caroli 55.70 9Kate Langley Unattached 16.51 -0.6 5Shavine Hodges Oxy Athletics 56.64 10Olivia Simmons Unattached 17.08 -0.8 6Makenzi Kopp JR Georgia 57.00 7Jhari Williams SO South Caroli 57.17 Women 100 Meter Dash 8Chelsea Zoller SO Georgia 57.28 Collegiate: 10.78 C6/2/1989 Dawn Sowell ---Briana Hardiman JR Arkansas Sta DNS NameYr School Finals ---Lynna Irby SO Georgia DNS Finals 1Makyla Stanley SR South Caroli 11.59 0.6 Women 800 Meter Run 2Brandee Presley FR Ole Miss 11.60 0.6 Collegiate: 1:59.10 C4/15/2017 Raevyn Rogers 3Chanice Porter Puma 11.67 1.1 NameYr School Finals 4Keturah Orji Atlanta Track Cl 11.68 1.1 Finals 5Milan Parks SR South Caroli 11.73 1.1 1Amber Tanner -

High-Resolution Mapping of Transcriptional Dynamics Across Tissue Development Reveals a Stable Mrna–Trna Interface
Downloaded from genome.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Research High-resolution mapping of transcriptional dynamics across tissue development reveals a stable mRNA–tRNA interface Bianca M. Schmitt,1,5 Konrad L.M. Rudolph,2,5 Panagiota Karagianni,3 Nuno A. Fonseca,2 Robert J. White,4 Iannis Talianidis,3 Duncan T. Odom,1 John C. Marioni,2 and Claudia Kutter1 1University of Cambridge, Cancer Research UK Cambridge Institute, Cambridge, CB2 0RE, United Kingdom; 2European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1SD, United Kingdom; 3B.S.R.C. Alexander Flemming, 16672, Vari, Athens, Greece; 4University of York, Department of Biology, Heslington, York, YO10 5DD, United Kingdom The genetic code is an abstraction of how mRNA codons and tRNA anticodons molecularly interact during protein synthesis; the stability and regulation of this interaction remains largely unexplored. Here, we characterized the expression of mRNA and tRNA genes quantitatively at multiple time points in two developing mouse tissues. We dis- covered that mRNA codon pools are highly stable over development and simply reflect the genomic background; in contrast, precise regulation of tRNA gene families is required to create the corresponding tRNA transcriptomes. The dynamic regulation of tRNA genes during development is controlled in order to generate an anticodon pool that closely corresponds to messenger RNAs. Thus, across development, the pools of mRNA codons and tRNA anticodons are invariant and highly correlated, revealing a stable molecular interaction interlocking transcription and translation. [Supplemental material is available for this article.] Transcription of the mammalian genome is divided among mul- differencesinexpressionofspecifictRNAgenesmaycontribute tiple RNA polymerases (Pol), each transcribing a nonoverlapping to tumorigenesis by favoring translation of cancer-promoting set of genes. -

UNIVERSITY of HAWAUN'wtytwln ~A~~7,0 CATALOG Z LIBRARY
• v, 44 /YlO.J- UNIVERSITY OF HAWAUN'WtYtWlN ~A~~7,0 CATALOG z LIBRARY en a: w Forty-fourth Annual SUMMER SESSION 1970 Catalog of Courses r • UNIVERSITY OF HAWAII BULLETIN VOL XLIX, NUMBER 2, MARCH 1970 The University of Hawaii Bulletin is pUblished in December, March, and twice in June by the University of Hawaii, Honolulu, Hawaii, U.S.A. Entered as second-class matter at the post office at Honolulu, Hawaii, November 14, 1921, under Act of Congress of August 24, 1912. PRICE 25 CENTS PLUS POSTAGE \ 1970 SUMMER CALENDAR JUNE SM T W T F S 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 JULy SMT W TF S .. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 AUGUST S M T W T F S 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 2 SEPTEMBER SMTW T F S 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 FIRST TERM ~JUNE IS-JULY 24. ~ June 10, 12, Wednesday, Friday .. Registration for UH students \.-.. and residents of Hawaii June IS, Monday _. -

DKFZ Postdoctoral Fellowships 2021 Hosting Group Information For
DKFZ Postdoctoral Fellowships 2021 Hosting group information for applicants Name of DKFZ research division/group: Division of Regulatory Genomics and Cancer Evolution (B270) Contact person: Duncan Odom Group homepage: https://www.dkfz.de/en/regulatorische-genomik/index.php Please visit our website for further information on our research and recent publications. RESEARCH PROFILE AND PROJECT TOPICS: Dr Odom’s laboratory studies how genetic sequence information shapes the cell's DNA regulatory landscape and thus the trajectory of cancer genome evolution. Our long-standing interest in interspecies analysis of matched functional genomic data has revealed the extensive and rapid turn-over of tissue-specific transcription factor binding, insulator elements, polymerase occupancies, and enhancer activities during organismal evolution. My laboratory has begun exploiting single-cell RNA-sequencing and large-scale whole genome sequencing in understanding molecular and cancer genome evolution. Recent high-profile studies from the Odom lab used single-cell transcriptional analysis to conclusively demonstrate that ageing results in substantial increases in cell-to-cell transcriptional variability and exploited chemical carcinogenesis in mouse liver to reveal how the persistence of DNA lesions profoundly shapes tumour genomes. First, we are currently interviewing for experimentally focused postdoctoral fellows to spearhead multiple diverse and interdisciplinary collaborations in single-cell analysis, functional genomics, and spatial transcriptomics with Gerstung, Stegle, Hoefer, and Goncalves laboratories at DKFZ. Such an appointment would offer unusual opportunities for an experimentally-focused postdoc to learn and deploy bioinformatics tools in collaboration with one or more world-leading computational biology laboratories. Second, we are seeking a postdoctoral researcher to spearhead a collaboration with Edith Heard's group at EMBL, focused on how X-chromosome dynamics can shape disease susceptibility in older individuals. -

The Tennessee Magazine
Ansearchin ' News, VOI.46, NO.3 Fa11 1999 rT THE TENNESSEE MAGAZINE THE TENNESSEE GENEALOGICAL SOCIETY 91 14 Davies Plantation Road on the histonc Davies Plantation Mailing Address: P. 0.Box 247, Brunswick, W38014-0247 Telephone: (901) 381-1447 5 OFFICER$ & BOARD MEMBES TGS Librarian Nelson Dickey ident JAMES E. BOB0 .or DOROTHY M. ROBERSON Dies After Extended Illness ing Librarian LORETTA BAILEY Nelson Dickey, librarian of the Tennessee Genealogical asurer FRANK PAESSLER Society for the past five years, died 23 June 1999 at a Mem- iness Manager JOHN WOODS phis hospital following an extended illness. He was 67. ording Secretary JO B. SMITH, Born 9 Oct 1931 in Jackson, Tenn., he was the son of responding Secretary SUE McDERMOTT George Hervey Dickey and Mayrne Huber Chumber of Milan. nbership Chairman SANDRA AUSTIN On 19 June 1957, Nelson rnanied Gladys Ann Ross in Milan. %tor of Sales DOUG GORDON They later moved to the Memphis area where he was vice :ctor of Certificates JANE PAESSLER president of Atlas Contractors, Inc. He was a Navy veteran ector at Large MARY ANN BELL of the Korean War, a member of the Germantown United Methodist Church choir, a Mason, Shriner, and member of ector at Large BETTY HUGHES ector of Surname Index JEAN CRAWFORD the Sons of the American Revolution. Nelson is survived by his wife; two daughters, Dara ector of Surname Index MARILYN VAN EYNDE Fields Dickey and Dawne Dickey Davis, both of Leesburg, Va.; and two grandchildren. Graveside rites were held at : Charles and Jane Paessler, Estelle Oakwood Cemetery in Milan on 24 June, and memorial Daniel. -

Paterson Institute for Cancer Research Scientific Report 2008 Contents
paterson institute for cancer research scientific report 2008 cover images: Main image supplied by Karim Labib and Alberto sanchez-Diaz (cell cycle Group). Budding yeast cells lacking the inn1 protein are unable to complete cytokinesis. these cells express a fusion of a green fluorescent protein to a marker of the plasma membrane, and have red fluorescent proteins attached to components of the spindle poles and actomyosin ring (sanchez-Diaz et al., nature cell Biology 2008; 10: 395). Additional images: front cover image supplied by Helen rushton, simon Woodcock and Angeliki Malliri (cell signalling Group). the image is of a mitotic spindle in fixed MDcK (Madin-Darby canine kidney) epithelial cells, which have been stained with an anti-beta tubulin antibody (green), DApi (blue) and an anti-centromere antibody (crest, red) which recognises the kinetochores of the chromosomes. the image was taken on the spinning disk confocal microscope using a 150 x lens. rear cover image supplied by Andrei ivanov and tim illidge (targeted therapy Group). Visualisation of tubulin (green) and quadripolar mitosis (DnA stained with DApi), Burkitt’s lymphoma namalwa cell after 10 Gy irradiation. issn 1740-4525 copyright 2008 © cancer research UK Paterson Institute for Cancer Research Scientific Report 2008 Contents 4 Director’s Introduction Researchers’ pages – Paterson Institute for Cancer Research 8 Crispin Miller Applied Computational Biology and Bioinformatics 10 Geoff Margison Carcinogenesis 12 Karim Labib Cell Cycle 14 Iain Hagan Cell Division 16 Nic Jones -

American Higher Education: Spatial Variation
AMERICAN HIGHER EDUCATION: SPATIAL VARIATION By PAUL LYMAN BUTT II Bachelor of Arts University of Wyoming Laramie, Wyoming 1965 Master of Arts University of Iowa Iowa City, Iowa 1972 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF EDUCATION July, 1975 - I ... ' ;· ., ' ~ ), ,J •t OKLAHOMA STATE UNIVERSITY UBRARY MAY .12 1976 AMERICAN HIGHER EDUCATION: SPATIAL VARIATION Thesis Approved: Dean of the Graduate College 938881 ii ACKNOWLEDGMENTS Many have assisted me in this study. The ideas and encouragement of my adviser, Dr. John F. Rooney, Jr., have been especially helpful. Appreciation is expressed to the other committee members, Professors Richard D. Hecock, Steven W. Tweedie, and Thomas A. Karman, for their helpful comments and suggestions in the preparation of this thesis; to Dr. Keith D. Harries for his useful critique; to the Oklahoma State University Cartographic Service personnel for their advice and for the addition of the cartographic "cosmetics.;" and to Mrs. Anna Gleason for the typing of the manuscript. I also want to acknowledge some of those who have helped to lighten the load along the way: Kathy, Olivia Newton-John, Kris and Rita, Bob Dylan, Annie Dillard, and Alex. Sincere thanks to each and every one. iii TABLE OF CONTENTS Chapter Page I. INTRODUCTION • 1 A Neglected Field • • 1 Statement of the Problem J Review of the Literature 4 Research Design 11 Indicators 11 Identification • 11 Interpretation • 12 Implications • 12 Justification of the Study 13 II• INDICATORS • 15 Components 15 Description, Definition and Intended Measurement. -

Register of Declared Private, Professional Commercial and Other Interests
REGISTER OF DECLARED PRIVATE, PROFESSIONAL COMMERCIAL AND OTHER INTERESTS Please refer to the attached guidance notes before completing this register entry. In addition to guidance on each section, examples of information required are also provided. Where you have no relevant interests in the relevant category, please enter ‘none’ in the register entry. Name: Michaela Frye Please list all MRC bodies you are a member of: E.g. Council, Strategy Board, Research Board, Expert Panel etc and your position (e.g. chair, member). Regenerative Medicine Research Committee member Main form of employment: Name of University and Department or other employing body (include location), and your position. University of Cambridge, Department of Genetics, Cancer Research UK Senior Fellow Research group/department web page: Provide a link to any relevant web pages for your research group or individual page on your organisation’s web site. http://www.stemcells.cam.ac.uk/research/affiliates/dr-frye http://www.gen.cam.ac.uk/research-groups/frye Please give details of any potential conflicts of interests arising out of the following: 1. Personal Remuneration: Including employment, pensions, consultancies, directorships, honoraria. See section 1 for further guidance. None 2. Shareholdings and Financial Interests in companies: Include the names of companies involved in medical/biomedical research, pharmaceuticals, biotechnology, healthcare provision and related fields where shareholdings or other financial interests. See section 2 for thresholds and further guidance. None 3. Research Income during current session (financial year) : Declare all research income from bodies supported by the MRC and research income from other sources above the limit of £50k per grant for the year.