Local Regulation of DNA Methylation by the Transcription Factor REST
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The REST/NRSF Pathway As a Central Mechanism in CNS Dysfunction
The REST/NRSF Pathway as a Central Mechanism in CNS Dysfunction Thesis submitted in accordance with the requirements of the University of Liverpool for the degree of Doctor in Philosophy by Alix Warburton April 2015 Disclaimer The data in this thesis is a result of my own work. The material collected for this thesis has not been presented, nor is currently being presented, either wholly or in part for any other degree or other qualification. All of the research, unless otherwise stated, was performed in the Department of Physiology and Department of Pharmacology, Institute of Translational Medicine, University of Liverpool. All other parties involved in the research presented here, and the nature of their contribution, are listed in the Acknowledgements section of this thesis. i Acknowledgements First and foremost, I would like to express my upmost gratitude to my primary and secondary supervisors Professor John Quinn (a.k.a Prof. Quinny) and Dr Jill Bubb for all of their support, guidance, wisdom (thank you Jill) and encouragement throughout my PhD; I could not have wished for a better pair. I am also extremely grateful to the BBSRC for funding my PhD project. I would also like to extend my thanks to Dr Graeme Sills for providing samples and assistance with my work on the SANAD epilepsy project, Dr Fabio Miyajima for offering his knowledge and knowhow on many occasions, Dr Gerome Breen for being a bioinformatics wizard and providing support on several projects, Dr Minyan Wang’s lab for their help and hospitality during my 3 month visit to Xi'an Jiaotong-Liverpool University, Dr Roshan Koron for assisting with the breast cancer study, Dr Chris Murgatroyd for his invaluable advice on ChIP and Professor Dan Rujescu’s lab for providing clinical samples and support with statistical analyses on the schizophrenia project. -
SM08 Programme
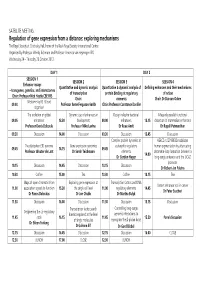
SATELLITE MEETING Regulation of gene expression from a distance: exploring mechanisms The Royal Society at Chicheley Hall, home of the Kavli Royal Society International Centre Organised by Professor Wendy Bickmore and Professor Veronica van Heyningen FRS Wednesday 24 – Thursday 25 October 2012 DAY 1 DAY 2 SESSION 1 SESSION 2 SESSION 3 SESSION 4 Enhancer assays Quantitative and dynamic analysis Quantitative & dynamic analysis of Defining enhancers and their mechanisms – transgenes, genetics, and interactomes of transcription protein binding at regulatory of action Chair: Professor Nick Hastie CBE FRS Chair: elements Chair: Dr Duncan Odom Welcome by RS & lead 09.00 Professor Anne Ferguson-Smith Chair: Professor Constance Bonifer organiser The evolution of global Dynamic use of enhancers in Design rules for bacterial Massively parallel functional 09.05 enhancers 13.30 development 09.00 enhancers 13.15 dissection of mammalian enhancers Professor Denis Duboule Professor Mike Levine Dr Roee Amit Dr Rupali Patwardhan 09.30 Discussion 14.00 Discussion 09.30 Discussion 13.45 Discussion Complex protein dynamics at HERC2 rs12913832 modulates The pluripotent 3D genome Gene expression genomics eukaryotic regulatory human pigmentation by attenuating 09.45 14.15 09.45 Professor Wouter de Laat Dr Sarah Teichmann elements chromatin-loop formation between a 14.00 Dr Gordon Hager long-range enhancer and the OCA2 promoter 10.15 Discussion 14.45 Discussion 10.15 Discussion Dr Robert-Jan Palstra 10.30 Coffee 15.00 Tea 10.30 Coffee 14.15 Tea Maps of open chromatin -

Mediator of DNA Damage Checkpoint 1 (MDC1) Is a Novel Estrogen Receptor Co-Regulator in Invasive 6 Lobular Carcinoma of the Breast 7 8 Evelyn K
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.16.423142; this version posted December 16, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Running Title: MDC1 co-regulates ER in ILC 2 3 Research article 4 5 Mediator of DNA damage checkpoint 1 (MDC1) is a novel estrogen receptor co-regulator in invasive 6 lobular carcinoma of the breast 7 8 Evelyn K. Bordeaux1+, Joseph L. Sottnik1+, Sanjana Mehrotra1, Sarah E. Ferrara2, Andrew E. Goodspeed2,3, James 9 C. Costello2,3, Matthew J. Sikora1 10 11 +EKB and JLS contributed equally to this project. 12 13 Affiliations 14 1Dept. of Pathology, University of Colorado Anschutz Medical Campus 15 2Biostatistics and Bioinformatics Shared Resource, University of Colorado Comprehensive Cancer Center 16 3Dept. of Pharmacology, University of Colorado Anschutz Medical Campus 17 18 Corresponding author 19 Matthew J. Sikora, PhD.; Mail Stop 8104, Research Complex 1 South, Room 5117, 12801 E. 17th Ave.; Aurora, 20 CO 80045. Tel: (303)724-4301; Fax: (303)724-3712; email: [email protected]. Twitter: 21 @mjsikora 22 23 Authors' contributions 24 MJS conceived of the project. MJS, EKB, and JLS designed and performed experiments. JLS developed models 25 for the project. EKB, JLS, SM, and AEG contributed to data analysis and interpretation. SEF, AEG, and JCC 26 developed and performed informatics analyses. MJS wrote the draft manuscript; all authors read and revised the 27 manuscript and have read and approved of this version of the manuscript. -

Intrinsic Specificity of DNA Binding and Function of Class II Bhlh
INTRINSIC SPECIFICITY OF BINDING AND REGULATORY FUNCTION OF CLASS II BHLH TRANSCRIPTION FACTORS APPROVED BY SUPERVISORY COMMITTEE Jane E. Johnson Ph.D. Helmut Kramer Ph.D. Genevieve Konopka Ph.D. Raymond MacDonald Ph.D. INTRINSIC SPECIFICITY OF BINDING AND REGULATORY FUNCTION OF CLASS II BHLH TRANSCRIPTION FACTORS by BRADFORD HARRIS CASEY DISSERTATION Presented to the Faculty of the Graduate School of Biomedical Sciences The University of Texas Southwestern Medical Center at Dallas In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY The University of Texas Southwestern Medical Center at Dallas Dallas, Texas December, 2016 DEDICATION This work is dedicated to my family, who have taught me pursue truth in all forms. To my grandparents for inspiring my curiosity, my parents for teaching me the value of a life in the service of others, my sisters for reminding me of the importance of patience, and to Rachel, who is both “the beautiful one”, and “the smart one”, and insists that I am clever and beautiful, too. Copyright by Bradford Harris Casey, 2016 All Rights Reserved INTRINSIC SPECIFICITY OF BINDING AND REGULATORY FUNCTION OF CLASS II BHLH TRANSCRIPTION FACTORS Publication No. Bradford Harris Casey The University of Texas Southwestern Medical Center at Dallas, 2016 Jane E. Johnson, Ph.D. PREFACE Embryonic development begins with a single cell, and gives rise to the many diverse cells which comprise the complex structures of the adult animal. Distinct cell fates require precise regulation to develop and maintain their functional characteristics. Transcription factors provide a mechanism to select tissue-specific programs of gene expression from the shared genome. -

Accompanies CD8 T Cell Effector Function Global DNA Methylation
Global DNA Methylation Remodeling Accompanies CD8 T Cell Effector Function Christopher D. Scharer, Benjamin G. Barwick, Benjamin A. Youngblood, Rafi Ahmed and Jeremy M. Boss This information is current as of October 1, 2021. J Immunol 2013; 191:3419-3429; Prepublished online 16 August 2013; doi: 10.4049/jimmunol.1301395 http://www.jimmunol.org/content/191/6/3419 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2013/08/20/jimmunol.130139 Material 5.DC1 References This article cites 81 articles, 25 of which you can access for free at: http://www.jimmunol.org/content/191/6/3419.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on October 1, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Global DNA Methylation Remodeling Accompanies CD8 T Cell Effector Function Christopher D. Scharer,* Benjamin G. Barwick,* Benjamin A. Youngblood,*,† Rafi Ahmed,*,† and Jeremy M. -

High-Resolution Mapping of Transcriptional Dynamics Across Tissue Development Reveals a Stable Mrna–Trna Interface
Downloaded from genome.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Research High-resolution mapping of transcriptional dynamics across tissue development reveals a stable mRNA–tRNA interface Bianca M. Schmitt,1,5 Konrad L.M. Rudolph,2,5 Panagiota Karagianni,3 Nuno A. Fonseca,2 Robert J. White,4 Iannis Talianidis,3 Duncan T. Odom,1 John C. Marioni,2 and Claudia Kutter1 1University of Cambridge, Cancer Research UK Cambridge Institute, Cambridge, CB2 0RE, United Kingdom; 2European Molecular Biology Laboratory, European Bioinformatics Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1SD, United Kingdom; 3B.S.R.C. Alexander Flemming, 16672, Vari, Athens, Greece; 4University of York, Department of Biology, Heslington, York, YO10 5DD, United Kingdom The genetic code is an abstraction of how mRNA codons and tRNA anticodons molecularly interact during protein synthesis; the stability and regulation of this interaction remains largely unexplored. Here, we characterized the expression of mRNA and tRNA genes quantitatively at multiple time points in two developing mouse tissues. We dis- covered that mRNA codon pools are highly stable over development and simply reflect the genomic background; in contrast, precise regulation of tRNA gene families is required to create the corresponding tRNA transcriptomes. The dynamic regulation of tRNA genes during development is controlled in order to generate an anticodon pool that closely corresponds to messenger RNAs. Thus, across development, the pools of mRNA codons and tRNA anticodons are invariant and highly correlated, revealing a stable molecular interaction interlocking transcription and translation. [Supplemental material is available for this article.] Transcription of the mammalian genome is divided among mul- differencesinexpressionofspecifictRNAgenesmaycontribute tiple RNA polymerases (Pol), each transcribing a nonoverlapping to tumorigenesis by favoring translation of cancer-promoting set of genes. -

DKFZ Postdoctoral Fellowships 2021 Hosting Group Information For
DKFZ Postdoctoral Fellowships 2021 Hosting group information for applicants Name of DKFZ research division/group: Division of Regulatory Genomics and Cancer Evolution (B270) Contact person: Duncan Odom Group homepage: https://www.dkfz.de/en/regulatorische-genomik/index.php Please visit our website for further information on our research and recent publications. RESEARCH PROFILE AND PROJECT TOPICS: Dr Odom’s laboratory studies how genetic sequence information shapes the cell's DNA regulatory landscape and thus the trajectory of cancer genome evolution. Our long-standing interest in interspecies analysis of matched functional genomic data has revealed the extensive and rapid turn-over of tissue-specific transcription factor binding, insulator elements, polymerase occupancies, and enhancer activities during organismal evolution. My laboratory has begun exploiting single-cell RNA-sequencing and large-scale whole genome sequencing in understanding molecular and cancer genome evolution. Recent high-profile studies from the Odom lab used single-cell transcriptional analysis to conclusively demonstrate that ageing results in substantial increases in cell-to-cell transcriptional variability and exploited chemical carcinogenesis in mouse liver to reveal how the persistence of DNA lesions profoundly shapes tumour genomes. First, we are currently interviewing for experimentally focused postdoctoral fellows to spearhead multiple diverse and interdisciplinary collaborations in single-cell analysis, functional genomics, and spatial transcriptomics with Gerstung, Stegle, Hoefer, and Goncalves laboratories at DKFZ. Such an appointment would offer unusual opportunities for an experimentally-focused postdoc to learn and deploy bioinformatics tools in collaboration with one or more world-leading computational biology laboratories. Second, we are seeking a postdoctoral researcher to spearhead a collaboration with Edith Heard's group at EMBL, focused on how X-chromosome dynamics can shape disease susceptibility in older individuals. -

Early B Cell Factor 1 Regulates B Cell Gene Networks by Activation, Repression, and Transcription- Independent Poising of Chromatin
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Immunity Resource Early B Cell Factor 1 Regulates B Cell Gene Networks by Activation, Repression, and Transcription- Independent Poising of Chromatin Thomas Treiber,1,3 Elizabeth M. Mandel,1,3 Sebastian Pott,2,3 Ildiko Gyo¨ ry,1,3 Sonja Firner,1 Edison T. Liu,2 and Rudolf Grosschedl1,* 1Max Planck Institute of Immunobiology, Department of Cellular and Molecular Immunology, Stuebeweg 51, 79108 Freiburg, Germany 2Genome Institute of Singapore, Cancer Biology and Pharmacology, 60 Biopolis Street, 138672 Singapore 3These authors contributed equally to this work *Correspondence: [email protected] DOI 10.1016/j.immuni.2010.04.013 SUMMARY (reviewed in Hardy et al., 2007; Murre, 2009). Pre-B cells express the pre-B cell receptor (pre-BCR) and further differentiate into The transcription factor early B cell factor-1 (Ebf1) is immature B cells that have undergone Ig light chain gene rear- a key determinant of B lineage specification and rangement and migrate from the bone marrow to the spleen. differentiation. To gain insight into the molecular Each of these B cell differentiation steps is dependent on basis of Ebf1 function in early-stage B cells, we the coordinated expression of cell-type-specific transcription combined a genome-wide ChIP sequencing analysis factors and activities of signaling pathways (reviewed in Mandel with gain- and loss-of-function transcriptome anal- and Grosschedl, 2010). Genetic ablation and complementation studies have demonstrated key roles for transcription factors yses. Among 565 genes that are occupied and tran- such as Ikaros, Pu.1, E2A, early B cell factor-1 (Ebf1), and scriptionally regulated by Ebf1, we identified large Pax5 (reviewed in Busslinger, 2004; Hagman and Lukin, 2006; sets involved in (pre)-B cell receptor and Akt Singh et al., 2007). -

Paterson Institute for Cancer Research Scientific Report 2008 Contents
paterson institute for cancer research scientific report 2008 cover images: Main image supplied by Karim Labib and Alberto sanchez-Diaz (cell cycle Group). Budding yeast cells lacking the inn1 protein are unable to complete cytokinesis. these cells express a fusion of a green fluorescent protein to a marker of the plasma membrane, and have red fluorescent proteins attached to components of the spindle poles and actomyosin ring (sanchez-Diaz et al., nature cell Biology 2008; 10: 395). Additional images: front cover image supplied by Helen rushton, simon Woodcock and Angeliki Malliri (cell signalling Group). the image is of a mitotic spindle in fixed MDcK (Madin-Darby canine kidney) epithelial cells, which have been stained with an anti-beta tubulin antibody (green), DApi (blue) and an anti-centromere antibody (crest, red) which recognises the kinetochores of the chromosomes. the image was taken on the spinning disk confocal microscope using a 150 x lens. rear cover image supplied by Andrei ivanov and tim illidge (targeted therapy Group). Visualisation of tubulin (green) and quadripolar mitosis (DnA stained with DApi), Burkitt’s lymphoma namalwa cell after 10 Gy irradiation. issn 1740-4525 copyright 2008 © cancer research UK Paterson Institute for Cancer Research Scientific Report 2008 Contents 4 Director’s Introduction Researchers’ pages – Paterson Institute for Cancer Research 8 Crispin Miller Applied Computational Biology and Bioinformatics 10 Geoff Margison Carcinogenesis 12 Karim Labib Cell Cycle 14 Iain Hagan Cell Division 16 Nic Jones -

Ebf1-Mediated Down-Regulation of Id2 and Id3 Is Essential for Specification of the B Cell Lineage
Ebf1-mediated down-regulation of Id2 and Id3 is essential for specification of the B cell lineage Melissa A. Thala, Thiago L. Carvalhoa,TiHea, Hyung-Gyoon Kima, Hua Gaob, James Hagmanb, and Christopher A. Kluga,1 aDepartment of Microbiology, The University of Alabama-Birmingham, Birmingham, AL 35294; and bIntegrated Department of Immunology, National Jewish Medical and Research Center, Denver, CO 80206 Edited by Cornelis Murre, University of California, San Diego, La Jolla, CA, and accepted by the Editorial Board November 7, 2008 (received for review March 13, 2008) Gene knockout experiments in mice have suggested a hierarchical of E47 (13) or E12 (14) in non-B-lineage cell lines, suggest that model of early B cell commitment wherein E2A proteins (E47 and E2A activity is essential upstream of Ebf1. Similarly, the Pax5 E12) activate early B cell factor (Ebf1), which in turn activates promoter is bound by Ebf1 based on EMSA and Ebf1 can expression of the B cell commitment factor, Pax5. In IL-7 receptor transactivate the Pax5 promoter in transient co-transfection alpha (IL-7R␣) knockout mice, B cell development is blocked before assays (15, 16). Ebf1 is also present in Pax5-deficient pro-B cells B-lineage commitment at the prepro-B cell stage in adult animals. derived from Pax5Ϫ/Ϫ adult mice (17), suggesting that Ebf1 may In IL-7R␣؊/؊ prepro-B cells, E47 is expressed and yet is insufficient participate in the activation of Pax5 expression. Complicating the to transcriptionally activate the putative downstream target gene, simple hierarchical model where E2A induces expression of Ebf1. -

EBF1 Drives Hallmark B Cell Gene Expression by Enabling the Interaction of PAX5 with the MLL H3K4 Methyltransferase Complex Charles E
www.nature.com/scientificreports OPEN EBF1 drives hallmark B cell gene expression by enabling the interaction of PAX5 with the MLL H3K4 methyltransferase complex Charles E. Bullerwell1, Philippe Pierre Robichaud1,2, Pierre M. L. Deprez1, Andrew P. Joy1, Gabriel Wajnberg1, Darwin D’Souza1,3, Simi Chacko1, Sébastien Fournier1, Nicolas Crapoulet1, David A. Barnett1,2, Stephen M. Lewis1,2 & Rodney J. Ouellette1,2* PAX5 and EBF1 work synergistically to regulate genes that are involved in B lymphocyte diferentiation. We used the KIS-1 difuse large B cell lymphoma cell line, which is reported to have elevated levels of PAX5 expression, to investigate the mechanism of EBF1- and PAX5-regulated gene expression. We demonstrate the lack of expression of hallmark B cell genes, including CD19, CD79b, and EBF1, in the KIS-1 cell line. Upon restoration of EBF1 expression we observed activation of CD19, CD79b and other genes with critical roles in B cell diferentiation. Mass spectrometry analyses of proteins co-immunoprecipitated with PAX5 in KIS-1 identifed components of the MLL H3K4 methylation complex, which drives histone modifcations associated with transcription activation. Immunoblotting showed a stronger association of this complex with PAX5 in the presence of EBF1. Silencing of KMT2A, the catalytic component of MLL, repressed the ability of exogenous EBF1 to activate transcription of both CD19 and CD79b in KIS-1 cells. We also fnd association of PAX5 with the MLL complex and decreased CD19 expression following silencing of KMT2A in other human B cell lines. These data support an important role for the MLL complex in PAX5-mediated transcription regulation. -

Logical Modeling of Lymphoid and Myeloid Cell Specification and Transdifferentiation
Logical modeling of lymphoid and myeloid cell specification and transdifferentiation Samuel Collombeta,1, Chris van Oevelenb,2, Jose Luis Sardina Ortegab,2, Wassim Abou-Jaoudéa, Bruno Di Stefanob,3, Morgane Thomas-Cholliera, Thomas Grafb,c,1, and Denis Thieffrya,1 aComputational Systems Biology Team, Institut de Biologie de l’Ecole Normale Supérieure, CNRS UMR8197, INSERM U1024, Ecole Normale Supérieure, Paris Sciences et Lettres Research University, 75005 Paris, France; bHematopoietic Stem Cells, Transdifferentiation, and Reprogramming Team, Gene Regulation, Stem Cells, and Cancer Program, Center for Genomic Regulation, Barcelona Institute for Biotechnology, 08003 Barcelona, Spain; and cUniversitat Pompeu Fabra, 08002 Barcelona, Spain Edited by Ellen V. Rothenberg, California Institute of Technology, Pasadena, CA, and accepted by Editorial Board Member Neil H. Shubin November 18, 2016 (received for review September 1, 2016) Blood cells are derived from a common set of hematopoietic stem for the transition from common myeloid progenitors (CMPs) to cells, which differentiate into more specific progenitors of the myeloid granulocyte-macrophage progenitors (GMPs), and mutation in and lymphoid lineages, ultimately leadingtodifferentiatedcells.This this gene can result in acute myeloid leukemia (6). Understanding developmental process is controlled by a complex regulatory network the molecular mechanisms by which such factors can induce cell- involving cytokines and their receptors, transcription factors, and fate decisions is of primary importance and might help in the chromatin remodelers. Using public data and data from our own mo- development of novel therapeutic strategies. lecular genetic experiments (quantitative PCR, Western blot, EMSA) or Computational modeling of regulatory networks is increasingly genome-wide assays (RNA-sequencing, ChIP-sequencing), we have recognized as a valuable approach to study cell-fate decisions.